Smoking-Associated Disordering of the Airway Basal Stem/Progenitor Cell Metabotype - PubMed (original) (raw)
Smoking-Associated Disordering of the Airway Basal Stem/Progenitor Cell Metabotype
Ruba S Deeb et al. Am J Respir Cell Mol Biol. 2016 Feb.
Abstract
The airway epithelium is a complex pseudostratified multicellular layer lining the tracheobronchial tree, functioning as the primary defense against inhaled environmental contaminants. The major cell types of the airway epithelium include basal, intermediate columnar, ciliated, and secretory. Basal cells (BCs) are the proliferating stem/progenitor population that differentiate into the other specialized cell types of the airway epithelium during normal turnover and repair. Given that cigarette smoke delivers thousands of xenobiotics and high levels of reactive molecules to the lung epithelial surface, we hypothesized that cigarette smoke broadly perturbs BC metabolism. To test this hypothesis, primary airway BCs were isolated from healthy nonsmokers (n = 11) and healthy smokers (n = 7) and assessed by global metabolic profiling by liquid chromatography-mass spectrometry. The analysis identified 52 significant metabolites in BCs differentially expressed between smokers and nonsmokers (P < 0.05). These changes included metabolites associated with redox pathways, energy production, and inflammatory processes. Notably, BCs from smokers exhibited altered levels of the key enzyme cofactors/substrates nicotinamide adenine dinucleotide, flavin adenine dinucleotide, acetyl coenzyme A, and membrane phospholipid levels. Consistent with the high burden of oxidants in cigarette smoke, glutathione levels were diminished, whereas 3-nitrotyrosine levels were increased, suggesting that protection of airway epithelial cells against oxidative and nitrosative stress is significantly compromised in smoker BCs. It is likely that this altered metabotype is a reflection of, and likely contributes to, the disordered biology of airway BCs consequent to the stress cigarette smoking puts on the airway epithelium.
Keywords: airway basal cells; cigarette smoke; metabolic profiling; oxidative stress; progenitor cells.
Figures
Figure 1.
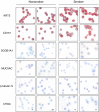
Characterization of primary human nonsmoker and smoker airway basal cells. Immunohistochemical characterization of cytopreps of primary human airway basal cells isolated using selective culture methods from large airway epithelial samples obtained by bronchoscopy from healthy nonsmokers and healthy smokers. Shown are cytokeratin 5 (KRT5; basal cell), tetraspanin-24 (CD151; basal cell), secretoglobin family 1A member 1 (SCGB1A1; secretory cell), mucin 5AC (MUC5AC; secretory cell), β-tubulin IV (ciliated cell), and chromogranin A (CHGA; neuroendocrine cell). Scale bar, 20 μm. Data shown are representative images from a single primary donor sample obtained for each phenotype.
Figure 2.
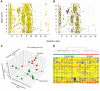
Untargeted liquid chromatography–mass spectrometry metabolite profiling of basal cells from nonsmokers and smokers. (A) Retention time versus mass depicting 2,920 aligned features detected with positive ion monitoring and quantified across all basal cell (BC) samples. (B) Retention time versus mass depicting 952 features observed in at least 50% of BC samples from at least one group. (C) Principal component analysis (PCA) showing that BCs from the nonsmoking group (green) cluster separately from the smoking group (red). Each point corresponds to a single sample. Each sample is connected by a vector to the groups’ centroid, representing the geometric center of each axis for all samples in that group. (D) Unsupervised hierarchical clustering analysis, with samples color-coded by phenotype, displays expression patterns and clustering of 952 quantified BC metabolites. Each column represents a single subject BC sample; horizontal bars represent feature intensity depicted as a heat map, ranging from cold (blue) to hot (red).
Figure 3.
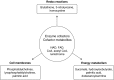
Smoking-affected metabolic pathways. Schematic diagram based on metabolic pathway analysis of differentially expressed metabolites between smokers and nonsmokers using Metaboanalyst software. Dominant pathways affected include redox reactions, cell membrane metabolism, energy metabolism, and enzyme cofactors and cofactor metabolites. CoA, coenzyme A; FAD, flavin adenine dinucleotide; NAD, nicotinamide adenine dinucleotide.
Figure 4.
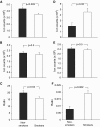
Basal cell redox status expressed as glutathione/oxidized glutathione (GSH/GSSG) and 3-nitrotyrosine/tyrosine (3-NT/Tyr) ratios. (A) Relative amounts of GSH accumulated in BCs from smokers compared with nonsmokers. (B) Relative amounts of GSSG accumulated in BCs from smokers compared with nonsmokers. (C) GSH/GSSG ratio comparing smokers with nonsmokers was calculated on a subject-by-subject basis by measuring relative ion counts for each molecule in each subject. (D) Relative amounts of 3-NT accumulated in BCs from smokers compared with nonsmokers. (E) Relative amounts of unmodified tyrosine accumulated in BCs from smokers compared with nonsmokers. (F) The 3-NT/Tyr ratio comparing smokers to nonsmokers was calculated on a subject-by-subject basis by measuring relative ion counts for each molecule in each subject. For all panels, data are presented as mean ± SEM, with statistics calculated by an unpaired Student’s t test.
Similar articles
- Increased expression of TROP2 in airway basal cells potentially contributes to airway remodeling in chronic obstructive pulmonary disease.
Liu Q, Li H, Wang Q, Zhang Y, Wang W, Dou S, Xiao W. Liu Q, et al. Respir Res. 2016 Nov 25;17(1):159. doi: 10.1186/s12931-016-0463-z. Respir Res. 2016. PMID: 27887617 Free PMC article. - Role of KRAS in regulating normal human airway basal cell differentiation.
Ogawa F, Walters MS, Shafquat A, O'Beirne SL, Kaner RJ, Mezey JG, Zhang H, Leopold PL, Crystal RG. Ogawa F, et al. Respir Res. 2019 Aug 9;20(1):181. doi: 10.1186/s12931-019-1129-4. Respir Res. 2019. PMID: 31399087 Free PMC article. - Gefitinib, an EGFR Tyrosine Kinase inhibitor, Prevents Smoke-Mediated Ciliated Airway Epithelial Cell Loss and Promotes Their Recovery.
Valencia-Gattas M, Conner GE, Fregien NL. Valencia-Gattas M, et al. PLoS One. 2016 Aug 17;11(8):e0160216. doi: 10.1371/journal.pone.0160216. eCollection 2016. PLoS One. 2016. PMID: 27532261 Free PMC article. - Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells.
Shaykhiev R, Crystal RG. Shaykhiev R, et al. Ann Am Thorac Soc. 2014 Dec;11 Suppl 5(Suppl 5):S252-8. doi: 10.1513/AnnalsATS.201402-049AW. Ann Am Thorac Soc. 2014. PMID: 25525728 Free PMC article. Review. - The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke.
Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. Faux SP, et al. Biomarkers. 2009 Jul;14 Suppl 1:90-6. doi: 10.1080/13547500902965047. Biomarkers. 2009. PMID: 19604067 Review.
Cited by
- Smoking and COVID-19: The Real Deal.
Eakin MN, Neptune E. Eakin MN, et al. Ann Am Thorac Soc. 2021 Oct;18(10):1610-1613. doi: 10.1513/AnnalsATS.202012-1537PS. Ann Am Thorac Soc. 2021. PMID: 33645465 Free PMC article. No abstract available. - Unlocking lung regeneration: insights into progenitor cell dynamics and metabolic control.
Yang J, Li Y, Huang Y, Chen H, Sui P. Yang J, et al. Cell Regen. 2024 Dec 16;13(1):31. doi: 10.1186/s13619-024-00212-y. Cell Regen. 2024. PMID: 39676102 Free PMC article. Review. - Mitochondrial Dysfunction in Airway Disease.
Prakash YS, Pabelick CM, Sieck GC. Prakash YS, et al. Chest. 2017 Sep;152(3):618-626. doi: 10.1016/j.chest.2017.03.020. Epub 2017 Mar 21. Chest. 2017. PMID: 28336486 Free PMC article. Review. - Hedgehog interacting protein (HHIP) represses airway remodeling and metabolic reprogramming in COPD-derived airway smooth muscle cells.
Li Y, Zhang L, Polverino F, Guo F, Hao Y, Lao T, Xu S, Li L, Pham B, Owen CA, Zhou X. Li Y, et al. Sci Rep. 2021 Apr 27;11(1):9074. doi: 10.1038/s41598-021-88434-x. Sci Rep. 2021. PMID: 33907231 Free PMC article. - Cartilaginous Extracellular Matrix Enriched with Human Gingival Mesenchymal Stem Cells Derived "Matrix Bound Extracellular Vesicles" Enabled Functional Reconstruction of Tracheal Defect.
Zeng T, Yuan P, Liang L, Zhang X, Zhang H, Wu W. Zeng T, et al. Adv Sci (Weinh). 2022 Jan;9(2):e2102735. doi: 10.1002/advs.202102735. Epub 2021 Nov 28. Adv Sci (Weinh). 2022. PMID: 34841733 Free PMC article.
References
- Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. - PubMed
- Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. - PubMed
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. - PubMed
- Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL087062/HL/NHLBI NIH HHS/United States
- P20 HL113443/HL/NHLBI NIH HHS/United States
- HL113443/HL/NHLBI NIH HHS/United States
- HL107882/HL/NHLBI NIH HHS/United States
- UL1 RR024143/RR/NCRR NIH HHS/United States
- HL087062/HL/NHLBI NIH HHS/United States
- R01 HL107882/HL/NHLBI NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical