Norepinephrine Reduces Reactive Oxygen Species (ROS) and DNA Damage in Ovarian Surface Epithelial Cells - PubMed (original) (raw)
Norepinephrine Reduces Reactive Oxygen Species (ROS) and DNA Damage in Ovarian Surface Epithelial Cells
Pooja R Patel et al. J Bioanal Biomed. 2015.
Abstract
Objective: To determine the role of norepinephrine (NE) on DNA damage and reactive oxygen species (ROS) generation in ovarian surface epithelial cells.
Method: Non-tumorigenic, immortalized ovarian surface epithelial cells were treated with NE, bleomycin, and bleomycin followed by NE. The comet assay was performed on each treatment group to determine the amount of single and double-strand breaks induced by treatments. ROS levels for each treatment group were measured using the H2DCF-DA fluorescence assay. Finally, RNA transcripts were measured for each treatment group with regards to the expression of DNA repair and oxidative stress genes.
Results: The mean tail moment of untreated cells was significantly greater than that of cells treated with NE (p=0.02). The mean tail moment of cells treated with bleomycin was significantly greater than that of cells treated with bleomycin followed by NE (p<0.01). Treatment with NE resulted in significantly less ROS generation than in untreated cells (p<0.01). NE treatment after hydrogen peroxide treatment resulted in a noticeable decrease in ROS generation. Genes associated with oxidative stress were upregulated in cells treated with bleomycin, however this upregulation was blunted when bleomycin-treated cells were treated subsequently with NE.
Conclusion: NE is associated with decreased DNA damage and ROS production in ovarian surface epithelial cells. This effect is protective in the presence of the oxidative-damaging agent bleomycin. These results suggest an additional physiologic role for the stress hormone NE, in protecting ovarian surface epithelial cells from oxidative stress.
Figures
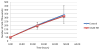
Figure 1
Cell viability assay. Presence of 10 uM norepinephrine does not affect cell viability of IOSE-29 ovarian surface epithelial cells. NE: Norepinephrine.
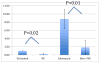
Figure 2
Comet assay results. This graph represents the levels of DNA damage with respect to untreated ovarian surface epithelial cells as a reference. DNA damage in cells treated with norepinephrine is significantly less than DNA damage in untreated cells (p=0.02). DNA damage in cells treated with bleomycin followed by norepinephrine is significantly less than DNA damage in cells treated with only bleomycin (p=0.01). NE: Norepinephrine; Bleo: Bleomycin; Bleo->NE: 30 minute treatment with bleomycin followed by 30 minute treatment with norepinephrine.
Figure 3
Comet assay images. Images of comet assay results of IOSE-29 cells. As expected, treatment with bleomycin resulted in larger comet tails, signifying greater DNA damage. Treatment with norepinephrine resulted in less tail, and subsequent treatment with norepinephrine after treatment with DNA damaging agent resulted in smaller tail. Bleo: Bleomycin; NE: Norepinephrine; Bleo->NE: 30 minute treatment with bleomycin followed by 30 minute treatment with norepinephrine.
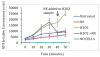
Figure 4
ROS generation measured by H2DCFDA. As expected, treatment with hydrogen peroxide resulted in greater ROS generation. Treatment with norepinephrine resulted in significantly less ROS generation than in untreated cells (p<0.01). Norepinephrine treatment after hydrogen peroxide treatment resulted in decreased ROS generation, however this different was not significant (p=0.14). H2O2: Hydrogen Peroxide; NE: Norepinephrine.
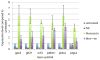
Figure 5
mRNA expression level of oxidative stress associated genes. All results are normalized to untreated cells. As expected, treatment with bleomycin resulted in increased expression of these genes when compared to untreated cells. Treatment with norepinephrine resulted in decreased expression of these genes when compared to untreated cells and treatment with norepinephrine after treatment with bleomycin resulted in decreased expression of these genes when compared with bleomycin treatment alone. Bleo: Bleomycin; NE: Norepinephrine; Bleo->NE: treatement with bleomycin for 30 minutes followed by treatment with norepinephrine for 30 minutes.
Figure 6
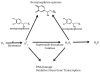
Proposed model of norepinephrine’s antioxidant effect in ovarian surface epithelial cells. Outlined above is the proposed pathway by which norepinephrine decreases ROS generation and subsequent DNA damage in ovarian surface epithelial cells. In this model, norepinephrine acts as a rapid ROS scavenger leading to a decrease in downstream DNA damage and reactive transcription of genes involved in the cellular response to oxidative stress.
Similar articles
- Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer.
Flaherty RL, Owen M, Fagan-Murphy A, Intabli H, Healy D, Patel A, Allen MC, Patel BA, Flint MS. Flaherty RL, et al. Breast Cancer Res. 2017 Mar 24;19(1):35. doi: 10.1186/s13058-017-0823-8. Breast Cancer Res. 2017. PMID: 28340615 Free PMC article. - Norepinephrine-induced apoptotic and hypertrophic responses in H9c2 cardiac myoblasts are characterized by different repertoire of reactive oxygen species generation.
Thakur A, Alam MJ, Ajayakumar MR, Ghaskadbi S, Sharma M, Goswami SK. Thakur A, et al. Redox Biol. 2015 Aug;5:243-252. doi: 10.1016/j.redox.2015.05.005. Epub 2015 May 29. Redox Biol. 2015. PMID: 26070033 Free PMC article. - DNA damage in human colonic mucosa cells induced by bleomycin and the protective action of vitamin E.
Woźniak K, Arabski M, Małecka-Panas E, Drzewoski J, Błasiak J. Woźniak K, et al. Cell Mol Biol Lett. 2004;9(1):31-45. Cell Mol Biol Lett. 2004. PMID: 15048149 - Effects of motexafin gadolinium on DNA damage and X-ray-induced DNA damage repair, as assessed by the Comet assay.
Donnelly ET, Liu Y, Paul TK, Rockwell S. Donnelly ET, et al. Int J Radiat Oncol Biol Phys. 2005 Jul 15;62(4):1176-86. doi: 10.1016/j.ijrobp.2005.04.014. Int J Radiat Oncol Biol Phys. 2005. PMID: 15990023 - Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells.
Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, Berg D, Berg U, Kunz L, Mayerhofer A. Saller S, et al. Endocrinology. 2012 Mar;153(3):1472-83. doi: 10.1210/en.2011-1769. Epub 2012 Jan 10. Endocrinology. 2012. PMID: 22234472
Cited by
- Nucleoside reverse transcriptase inhibitor-induced rat oocyte dysfunction and low fertility mediated by autophagy.
Tang L, Yang S, Wang H, Gu H, Xia X, Feng Y, Yang Z, Zhao S, Su C, Su Z, Wang K. Tang L, et al. Oncotarget. 2017 Dec 13;9(3):3895-3907. doi: 10.18632/oncotarget.23243. eCollection 2018 Jan 9. Oncotarget. 2017. PMID: 29423092 Free PMC article. - Daphnetin, a natural coumarin averts reserpine-induced fibromyalgia in mice: modulation of MAO-A.
Singh L, Kaur A, Singh AP, Bhatti R. Singh L, et al. Exp Brain Res. 2021 May;239(5):1451-1463. doi: 10.1007/s00221-021-06064-1. Epub 2021 Mar 7. Exp Brain Res. 2021. PMID: 33677656 - Norepinephrine-Induced DNA Damage in Ovarian Cancer Cells.
Lamboy-Caraballo R, Ortiz-Sanchez C, Acevedo-Santiago A, Matta J, N A Monteiro A, N Armaiz-Pena G. Lamboy-Caraballo R, et al. Int J Mol Sci. 2020 Mar 24;21(6):2250. doi: 10.3390/ijms21062250. Int J Mol Sci. 2020. PMID: 32213975 Free PMC article. - Impact of norepinephrine on immunity and oxidative metabolism in sepsis.
Thoppil J, Mehta P, Bartels B, Sharma D, Farrar JD. Thoppil J, et al. Front Immunol. 2023 Nov 7;14:1271098. doi: 10.3389/fimmu.2023.1271098. eCollection 2023. Front Immunol. 2023. PMID: 38022663 Free PMC article. Review. - Nerves in gastrointestinal cancer: from mechanism to modulations.
Vaes N, Idris M, Boesmans W, Alves MM, Melotte V. Vaes N, et al. Nat Rev Gastroenterol Hepatol. 2022 Dec;19(12):768-784. doi: 10.1038/s41575-022-00669-9. Epub 2022 Sep 2. Nat Rev Gastroenterol Hepatol. 2022. PMID: 36056202 Review.
References
- Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. - PubMed
LinkOut - more resources
Full Text Sources