Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain - PubMed (original) (raw)
. 2015 Jul 28;112(30):9484-9.
doi: 10.1073/pnas.1508545112. Epub 2015 Jul 13.
Yi Zhou 2, Ryan P Stadel 3, Jonathan Moss 4, Jing Hui A Yong 5, Shiori Ito 6, Nicholas K Kawasaki 6, Alexander T Phan 6, Justin H Oh 6, Nikhil Modak 6, Randall R Reed 7, Nicolas Toni 4, Hongjun Song 8, Guo-li Ming 9
Affiliations
- PMID: 26170290
- PMCID: PMC4522763
- DOI: 10.1073/pnas.1508545112
Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain
Gerald J Sun et al. Proc Natl Acad Sci U S A. 2015.
Abstract
In a classic model of mammalian brain formation, precursors of principal glutamatergic neurons migrate radially along radial glia fibers whereas GABAergic interneuron precursors migrate tangentially. These migration modes have significant implications for brain function. Here we used clonal lineage tracing of active radial glia-like neural stem cells in the adult mouse dentate gyrus and made the surprising discovery that proliferating neuronal precursors of glutamatergic granule neurons exhibit significant tangential migration along blood vessels, followed by limited radial migration. Genetic birthdating and morphological and molecular analyses pinpointed the neuroblast stage as the main developmental window when tangential migration occurs. We also developed a partial "whole-mount" dentate gyrus preparation and observed a dense plexus of capillaries, with which only neuroblasts, among the entire population of progenitors, are directly associated. Together, these results provide insight into neuronal migration in the adult mammalian nervous system.
Keywords: adult neurogenesis; hippocampus; lineage tracing; migration; vascular niche.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
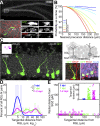
Tangential distribution of newborn granule neurons away from their parental RGLs of origin in the adult dentate gyrus as revealed by in vivo clonal analysis. (A) Sample projected confocal image of an Ascl1 CreERT2 ;Rosa-YFP _f/+_–labeled RGL in the dentate gyrus at 1 dpi. As shown in a high-magnification image (Bottom), this GFAP+ RGL (closed arrowheads) underwent an asymmetric division to produce a GFAP− neuronal progeny (open arrowheads; on a different focal plane). [Scale bars, 100 μm (Top) and 10 μm (Bottom).] (B) Computational simulations of the nearest distances between two cells in the 3D dentate gyrus space. Each colored line represents the reverse cumulative distribution of the nearest cell distances from a simulation with a given number of total cells. (C) (Left) Sample projection confocal image of a labeled clone in an Ascl1 CreERT2 ;Rosa-YFP f/+ mouse at 1 mpi (Movie S1) showing tangential distribution of Prox1+NeuN+ mature glutamatergic granule neurons and their parental GFAP+ RGL. (Inset) Two-dimensional SGZ plane projection in 200-μm-square windows of all clonally related cell locations. The RGL is represented as the center open circle; neural progeny are represented as closed circles. (Right, Top) Schematic diagram for measurements of tangential and radial distances of neuronal progeny from the parental RGL. (Right, Bottom) Enlarged confocal images for a GFAP+ RGL (1) and Prox1+NeuN+ mature neuronal progeny (2). (Scale bars, 10 µm.) GCL, granule cell layer. (D) Distribution plot of the tangential distance between a labeled RGL and its progeny within each clone at 3 dpi, 7 dpi, and 1–2 mpi (*P < 0.05; two-sample Kolmogorov–Smirnov test statistic: 0.85 for 3 vs. 7 dpi; 0.66 for 3 dpi vs. 1–2 mpi). Raw distributions are shown as bar graphs; curved lines correspond to smoothed distributions. (E) Dot plot of the tangential versus radial distance from the parental RGL for each neural progeny in all labeled RGL-containing clones at 3 dpi, 7 dpi, and 1–2 mpi. (Inset) Tangential distance of each neural progeny from its parental RGL.
Fig. 2.
Developmental stage-specific tangential distribution of newborn neural progeny. (A) Summary of molecular markers used for identification of each cell type during adult hippocampal neurogenesis. Newborn cells are generated from GFAP+Nestin+ RGLs that undergo asymmetric neuronal divisions, which develop into Tbr2+ intermediate progenitor cells with short, multipolar processes within 3 d of birth. Within 3–7 d, newborn cells possess long, bipolar processes and elongated somas in a Tbr2+/−DCX+ neuroblast stage before penetrating the granule cell layer and becoming a polarized Prox1+DCX+ immature neuron with axon and dendrite. Over the next month, newborn cells mature into Prox1+NeuN+ neurons with spiny dendrites and long axons that project to CA3. See also Table S1. (B) Summary of the tangential distance of each neural progeny from its parental RGL at each developmental stage. IN, immature neuron; N, mature granule neuron; NB, neuroblast. Values represent mean ± SEM (*P < 0.01; #P ∼ 1; Wilcoxon rank-sum test with Bonferroni correction for multiple comparisons; test statistic, z = −5.58 and −0.72, respectively). (C) Histogram and cumulative distribution plot of the maximum distance between clonally related neural progeny for all clones, including non–RGL-containing clones. See Fig. S1 for a 2D SGZ plane projection of representative clones (*P < 0.1; two-sample Kolmogorov–Smirnov test statistic: 0.38).
Fig. S1.
Tangential distribution among newborn neural progeny at 3 dpi, 7 dpi, and 1–2 mpi in the adult dentate gyrus. Two-dimensional SGZ plane projection of all RGL-containing clones containing at least IPCs at (A) 3 dpi, (B) 7 dpi, and (C) 1–2 mpi, plotted in a 300-μm-square window. RGLs are represented as open circles; neural progeny are represented as closed circles. Different time points or developmental stages are encoded by color. Clones that lacked RGLs at 1–2 mpi are also plotted in gray boxes and included in the histogram in Fig. 2_C_.
Fig. 3.
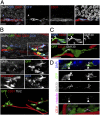
Close association between tangentially migrating neuroblasts and blood vessels in the adult dentate gyrus. (A) Sample confocal images (Left) and 3D rendering (Far Right) of a CFP+ clone at 7 dpi with a neuroblast whose soma and tangential process closely associated with a CD31+ blood vessel. (B) Sample confocal image and 3D rendering of a GFP+ clone at 7 dpi containing a parental RGL and dispersed Tbr2+DCX+ neuroblasts (open arrowheads) in close association with CD31+ blood vessels (Movie S2). Note that CD31 and Tbr2 shared the same channel due to limited availability of channels but could be distinguished by different morphology (Tbr2, nuclear staining; CD31, tubular staining). (C) Sample confocal image and 3D rendering of a GFP+ clone at 7 dpi with a neuroblast whose tangential process extended along a blood vessel and contained polarized GM130+ Golgi apparatus (closed arrowheads) at its base, proximal to the cell soma. (D) Sample confocal image and 3D rendering of GFP+ neuroblasts at 7 dpi with polarized γ-tubulin+ centrosomes (closed arrowheads) and GM130+ Golgi apparatus (open arrowheads) and in close association with CD31+ blood vessels. (Scale bars, 10 μm.)
Fig. 4.
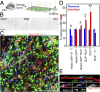
Direct contact between tangentially migrating neuroblasts and blood vessels in the adult dentate gyrus. (A) (Left) Quantification of the distribution of various labeled precursors in close association with the vasculature at 7 dpi. Values represent mean ± SEM. (Right) Summaries of precursor cell molecular marker expression and the percentage of a given cell subtype in close association with the vasculature. BV, blood vessel. (B) Three-dimensional reconstruction, from serial electron microscopic sections, of a GFP+ SGZ neuronal precursor (NP1; closed arrowhead) extending a tangential process along a blood vessel. The immunofluorescence-identified, immunoperoxidase-labeled cell (Top) sits alongside another neuronal precursor (NP2; open arrowhead) and apposes the blood vessel with both its tangential process (Middle Left) and its cell body (Top Right; arrowhead). (Scale bars, 5 µm.)
Fig. 5.
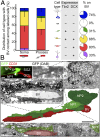
Neuroblast–vasculature interaction using a partial whole-mount dentate gyrus SGZ preparation. (A) Schematic illustration of the partial whole-mount SGZ preparation (Movie S3). (B) CD31+ blood vessels using a whole-mount preparation visualized in SGZ (Left) and GCL (Right). (Scale bar, 100 μm.) (C) Sample projected confocal image of Nestin-GFP tissue from a partial whole-mount preparation immunostained for Tbr2, CD31, and DCX (Movie S4). (Scale bar, 100 μm.) (D) Quantification of the vascular association of different cell types expressing combinations of Nestin-GFP, Tbr2, and DCX using a partial whole-mount preparation to view the population of progenitors in the SGZ. Association was defined as having cell soma on a blood vessel and compared with simulated random distributions of the same numbers of cells in the same space. Values represent means ± SEM [n = 3 animals; *P < 0.05; **_P_ < 0.01; one-tailed unpaired Student’s _t_ test, observed > simulated; Tbr2+: t(4) = 2.20; Tbr2+DCX+: t(4) = 2.44; DCX+ (neuroblast): t(4) = 3.76]. (E) Sample confocal image and 3D rendering of a Tbr2+DCX+ neuroblast migrating on a CD31+ blood vessel with polarized GM130+ Golgi apparatus (closed arrowhead) from a partial whole-mount preparation in a Nestin-GFP mouse. (Scale bars, 10 μm.)
Fig. 6.
Two-step model for neuronal migration during adult hippocampal neurogenesis. During adult hippocampal neurogenesis, radial glia-like cells give rise to Tbr2+ intermediate progenitor cells within 3 d. In the next 4 d, the cells become DCX+ proliferating neuroblasts that extend long processes tangential to the SGZ and contact blood vessels directly. During this phase, neuroblasts use blood vessels as a substrate to migrate tangentially away from their parental RGL. After 7 d, newborn neural progeny extend radial dendritic processes and develop into NeuN+Prox1+ dentate granule neurons and exhibit limited radial migration through the granule cell layer. (Lower) Global-view illustration of RGLs, neural progeny, and vasculature in the adult SGZ.
Similar articles
- The microtubule destabilizing protein stathmin controls the transition from dividing neuronal precursors to postmitotic neurons during adult hippocampal neurogenesis.
Boekhoorn K, van Dis V, Goedknegt E, Sobel A, Lucassen PJ, Hoogenraad CC. Boekhoorn K, et al. Dev Neurobiol. 2014 Dec;74(12):1226-42. doi: 10.1002/dneu.22200. Epub 2014 Jun 20. Dev Neurobiol. 2014. PMID: 24909416 - Long-Term Fate Mapping Using Conditional Lentiviral Vectors Reveals a Continuous Contribution of Radial Glia-Like Cells to Adult Hippocampal Neurogenesis in Mice.
Aelvoet SA, Pascual-Brazo J, Libbrecht S, Reumers V, Gijsbers R, Van den Haute C, Baekelandt V. Aelvoet SA, et al. PLoS One. 2015 Nov 23;10(11):e0143772. doi: 10.1371/journal.pone.0143772. eCollection 2015. PLoS One. 2015. PMID: 26600383 Free PMC article. - A vascular perspective on neuronal migration.
Segarra M, Kirchmaier BC, Acker-Palmer A. Segarra M, et al. Mech Dev. 2015 Nov;138 Pt 1:17-25. doi: 10.1016/j.mod.2015.07.004. Epub 2015 Jul 17. Mech Dev. 2015. PMID: 26192337 Review. - Modes and mishaps of neuronal migration in the mammalian brain.
Métin C, Vallee RB, Rakic P, Bhide PG. Métin C, et al. J Neurosci. 2008 Nov 12;28(46):11746-52. doi: 10.1523/JNEUROSCI.3860-08.2008. J Neurosci. 2008. PMID: 19005035 Free PMC article. Review.
Cited by
- Abrogation of atypical neurogenesis and vascular-derived EphA4 prevents repeated mild TBI-induced learning and memory impairments.
Greer K, Basso EKG, Kelly C, Cash A, Kowalski E, Cerna S, Ocampo CT, Wang X, Theus MH. Greer K, et al. Sci Rep. 2020 Sep 21;10(1):15374. doi: 10.1038/s41598-020-72380-1. Sci Rep. 2020. PMID: 32958852 Free PMC article. - Neural Progenitor Cells and the Hypothalamus.
Makrygianni EA, Chrousos GP. Makrygianni EA, et al. Cells. 2023 Jul 11;12(14):1822. doi: 10.3390/cells12141822. Cells. 2023. PMID: 37508487 Free PMC article. Review. - Stem Cell-Based Therapies: What Interventional Radiologists Need to Know.
Yu H, Commander CW, Stavas JM. Yu H, et al. Semin Intervent Radiol. 2021 Nov 24;38(5):523-534. doi: 10.1055/s-0041-1736657. eCollection 2021 Dec. Semin Intervent Radiol. 2021. PMID: 34853498 Free PMC article. Review. - Deciphering the neuroprotective and neurogenic potential of soluble amyloid precursor protein alpha (sAPPα).
Dar NJ, Glazner GW. Dar NJ, et al. Cell Mol Life Sci. 2020 Jun;77(12):2315-2330. doi: 10.1007/s00018-019-03404-x. Epub 2020 Jan 20. Cell Mol Life Sci. 2020. PMID: 31960113 Free PMC article. Review. - Regenerative Potential of Carbon Monoxide in Adult Neural Circuits of the Central Nervous System.
Jung E, Koh SH, Yoo M, Choi YK. Jung E, et al. Int J Mol Sci. 2020 Mar 25;21(7):2273. doi: 10.3390/ijms21072273. Int J Mol Sci. 2020. PMID: 32218342 Free PMC article. Review.
References
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: Non-radial migration in the mammalian forebrain. Nat Neurosci. 2001;4(Suppl):1177–1182. - PubMed
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007814/GM/NIGMS NIH HHS/United States
- MH105128/MH/NIMH NIH HHS/United States
- NS048271/NS/NINDS NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- NS047344/NS/NINDS NIH HHS/United States
- R01 MH105128/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases