Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients - PubMed (original) (raw)
Clinical Trial
. 2015 Dec;30(12):2019-26.
doi: 10.1093/ndt/gfv277. Epub 2015 Jul 13.
Affiliations
- PMID: 26175145
- PMCID: PMC4656038
- DOI: 10.1093/ndt/gfv277
Clinical Trial
Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients
Steven N Fishbane et al. Nephrol Dial Transplant. 2015 Dec.
Abstract
Background: Administration of ferric pyrophosphate citrate (FPC, Triferic™) via hemodialysate may allow replacement of ongoing uremic and hemodialysis-related iron losses. FPC donates iron directly to transferrin, bypassing the reticuloendothelial system and avoiding iron sequestration.
Methods: Two identical Phase 3, randomized, placebo-controlled trials (CRUISE 1 and 2) were conducted in 599 iron-replete chronic hemodialysis patients. Patients were dialyzed with dialysate containing 2 µM FPC-iron or standard dialysate (placebo) for up to 48 weeks. Oral or intravenous iron supplementation was prohibited, and doses of erythropoiesis-stimulating agents were held constant. The primary efficacy end point was the change in hemoglobin (Hgb) concentration from baseline to end of treatment (EoT). Secondary end points included reticulocyte hemoglobin content (CHr) and serum ferritin.
Results: In both trials, Hgb concentration was maintained from baseline to EoT in the FPC group but decreased by 0.4 g/dL in the placebo group (P < 0.001, combined results; 95% confidence interval [CI] 0.2-0.6). Placebo treatment resulted in significantly larger mean decreases from baseline in CHr (-0.9 pg versus -0.4 pg, P < 0.001) and serum ferritin (-133.1 µg/L versus -69.7 µg/L, P < 0.001) than FPC treatment. The proportions of patients with adverse and serious adverse events were similar in both treatment groups.
Conclusions: FPC delivered via dialysate during hemodialysis replaces iron losses, maintains Hgb concentrations, does not increase iron stores and exhibits a safety profile similar to placebo. FPC administered by hemodialysis via dialysate represents a paradigm shift in delivering maintenance iron therapy to hemodialysis patients.
Trial registration: ClinicalTrials.gov NCT01320202 NCT01322347.
Keywords: anemia; erythropoiesis-stimulating agent; ferric pyrophosphate citrate; hemodialysis; iron.
© The Author 2015. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
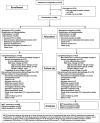
FIGURE 1:
Patient disposition—CONSORT diagram. ESA, erythropoiesis-stimulating agent; FPC, ferric pyrophosphate citrate; Hgb, hemoglobin; IV, intravenous; MITT, modified intent to treat.
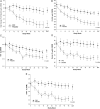
FIGURE 2:
Mean change in Hgb concentration (A), ferritin concentration (B), CHr (C), serum iron concentration (D) and TSAT (E) from baseline during Stage 2-CRUISE 1 and 2 studies combined (MITT population). Values other than EoT are based on an LOCF analysis. Bars indicate SEM. For Hgb, P-value is from an analysis of covariance with baseline Hgb as the covariate. For all other parameters, P-value is from a Wilcoxon rank-sum test. CHr, reticulocyte Hgb content; EoT, end of treatment; Fe, iron; FPC, ferric pyrophosphate citrate; Hgb, hemoglobin; LOCF, last post-baseline observation carried forward; MITT, modified intent to treat (FPC, N = 290; placebo, N = 295); TSAT, transferrin saturation.
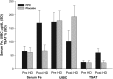
FIGURE 3:
Mean change from pre-hemodialysis to post-hemodialysis in serum iron, UIBC and TSAT—CRUISE 1 and 2 studies combined (composite data for Stage 2, MITT population). Fe, iron; FPC, ferric pyrophosphate citrate; HD, hemodialysis; TSAT, transferrin saturation; UIBC, unsaturated iron-binding capacity; MITT, modified intent to treat (FPC, N = 290; placebo_, N =_ 295).
Comment in
- Ferric pyrophosphate: good things come to those who wait?
Fell LH, Fliser D, Heine GH. Fell LH, et al. Nephrol Dial Transplant. 2015 Dec;30(12):1942-4. doi: 10.1093/ndt/gfv287. Epub 2015 Jul 21. Nephrol Dial Transplant. 2015. PMID: 26203048 Free PMC article.
Similar articles
- Ferric Pyrophosphate Citrate: A Novel Iron Replacement Agent in Patients Undergoing Hemodialysis.
Shah HH, Hazzan AD, Fishbane S. Shah HH, et al. Semin Nephrol. 2016 Mar;36(2):124-9. doi: 10.1016/j.semnephrol.2016.02.007. Semin Nephrol. 2016. PMID: 27236134 Review. - Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients.
Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, Besarab A. Gupta A, et al. Kidney Int. 2015 Nov;88(5):1187-94. doi: 10.1038/ki.2015.203. Epub 2015 Jul 8. Kidney Int. 2015. PMID: 26154926 Free PMC article. Clinical Trial. - Pharmacokinetics of ferric pyrophosphate citrate administered via dialysate and intravenously to pediatric patients on chronic hemodialysis.
Pratt RD, Grimberg S, Zaritsky JJ, Warady BA. Pratt RD, et al. Pediatr Nephrol. 2018 Nov;33(11):2151-2159. doi: 10.1007/s00467-018-4014-3. Epub 2018 Jul 12. Pediatr Nephrol. 2018. PMID: 30003313 Free PMC article. Clinical Trial. - Ferric pyrophosphate citrate as an iron replacement agent for patients receiving hemodialysis.
Fishbane S, Shah HH. Fishbane S, et al. Hemodial Int. 2017 Jun;21 Suppl 1:S104-S109. doi: 10.1111/hdi.12554. Epub 2017 Apr 3. Hemodial Int. 2017. PMID: 28371161 Review. - Ferric pyrophosphate citrate for parenteral administration of maintenance iron: structure, mechanism of action, clinical efficacy and safety.
Fishbane S, Ganz T, Pratt RD. Fishbane S, et al. Curr Med Res Opin. 2022 Aug;38(8):1417-1429. doi: 10.1080/03007995.2022.2092373. Epub 2022 Jul 7. Curr Med Res Opin. 2022. PMID: 35726771
Cited by
- Managing Anemia: Point of Convergence for Heart Failure and Chronic Kidney Disease?
Buliga-Finis ON, Ouatu A, Tanase DM, Gosav EM, Seritean Isac PN, Richter P, Rezus C. Buliga-Finis ON, et al. Life (Basel). 2023 Jun 1;13(6):1311. doi: 10.3390/life13061311. Life (Basel). 2023. PMID: 37374094 Free PMC article. Review. - Individualized anemia management enhanced by ferric pyrophosphate citrate protocol.
Chait Y, Nathanson BH, Germain MJ. Chait Y, et al. Sci Rep. 2022 Nov 22;12(1):20122. doi: 10.1038/s41598-022-23262-1. Sci Rep. 2022. PMID: 36418453 Free PMC article. - Ethnicity evaluation of ferric pyrophosphate citrate among Asian and Non-Asian populations: a population pharmacokinetics analysis.
Zhang L, Gan L, Li K, Xie P, Tan Y, Wei G, Yuan X, Pratt R, Zhou Y, Hui AM, Fang Y, Zuo L, Zheng Q. Zhang L, et al. Eur J Clin Pharmacol. 2022 Sep;78(9):1421-1434. doi: 10.1007/s00228-022-03328-9. Epub 2022 Jun 17. Eur J Clin Pharmacol. 2022. PMID: 35711066 Free PMC article. - Institutional Usage of Ferric Pyrophosphate Citrate (FPC) Delivered Via Dialysate in Reducing Erythropoiesis Stimulating Agents (ESAs) and IV Iron Cost.
Wang S, DellaFera L, Dhanani L, Malone B, Dutka P, Akerman M, Masani N. Wang S, et al. Hosp Pharm. 2022 Jun;57(3):355-358. doi: 10.1177/00185787211032363. Epub 2021 Jul 26. Hosp Pharm. 2022. PMID: 35615489 Free PMC article. - Pharmacokinetics and Safety of Ferric Pyrophosphate Citrate in Chinese Subjects with and without Hemodialysis-Dependent Stage 5 Chronic Kidney Disease.
Gan L, Xie P, Tan Y, Wei G, Yuan X, Lu Z, Pratt R, Zhou Y, Hui AM, Li K, Fang Y, Zuo L. Gan L, et al. Drugs R D. 2022 Jun;22(2):119-129. doi: 10.1007/s40268-022-00384-5. Epub 2022 Apr 5. Drugs R D. 2022. PMID: 35380419 Free PMC article. Clinical Trial.
References
- Sargent JA, Acchiardo SR. Iron requirements in hemodialysis. Blood Purif 2004; 22: 112–1123 - PubMed
- Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010; 116: 4754–4761 - PubMed
- Bailie GR, Larkina M, Goodkin DA et al. . Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2013; 28: 2570–2579 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical