Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy - PubMed (original) (raw)
Review
Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy
Mark T Waddingham et al. World J Diabetes. 2015.
Abstract
Diabetes mellitus significantly increases the risk of cardiovascular disease and heart failure in patients. Independent of hypertension and coronary artery disease, diabetes is associated with a specific cardiomyopathy, known as diabetic cardiomyopathy (DCM). Four decades of research in experimental animal models and advances in clinical imaging techniques suggest that DCM is a progressive disease, beginning early after the onset of type 1 and type 2 diabetes, ahead of left ventricular remodeling and overt diastolic dysfunction. Although the molecular pathogenesis of early DCM still remains largely unclear, activation of protein kinase C appears to be central in driving the oxidative stress dependent and independent pathways in the development of contractile dysfunction. Multiple subcellular alterations to the cardiomyocyte are now being highlighted as critical events in the early changes to the rate of force development, relaxation and stability under pathophysiological stresses. These changes include perturbed calcium handling, suppressed activity of aerobic energy producing enzymes, altered transcriptional and posttranslational modification of membrane and sarcomeric cytoskeletal proteins, reduced actin-myosin cross-bridge cycling and dynamics, and changed myofilament calcium sensitivity. In this review, we will present and discuss novel aspects of the molecular pathogenesis of early DCM, with a special focus on the sarcomeric contractile apparatus.
Keywords: Diabetes; Insulin resistance; Myocardium; Prediabetes; Protein kinase C; Rho kinase; Sarcomere.
Figures
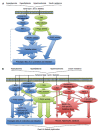
Figure 1
Cellular signaling pathways. A: Cellular signaling pathways driving contractile dysfunction in early diabetic cardiomyopathy; B: Cellular signaling pathways involved in the development of overt LV diastolic dysfunction in diabetes. ANGII: Angiotensin II; DAG: Diacylglycerol; NADPH: Nicotinamide adenine dinucleotide phosphate; PKC: Protein kinase C; RAAS: Renin angiotensin aldosterone system; ROCK: Rho kinase; AGE: Advanced glycation end products; ANG1-7: Angiotensin 1-7; ETC: Electron transport chain; iNOS: Inducible nitric oxide synthase; NF-κB: Nuclear factor-κB; NO: Nitric oxide; SOD: Superoxide dismutase; LV: Left ventricle.
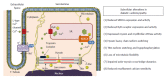
Figure 2
Subcellular alterations in various compartments of the cardiomyocyte in diabetic cardiomyopathy. PLB: Phospholamban; SERCA: Sarcoplasmic reticulum Ca2+-ATPase; SR: Sarcoplasmic reticulum; RyR: Ryanodine receptor.
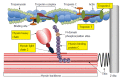
Figure 3
Illustration of the cardiac sarcomere indicating the location of actin thin-filament and myosin thick-filament accessory proteins involved in the regulation of actin-myosin cross-bridge dynamics and kinetics. C0-C10: Immunoglobulin-like and fibronectin-like domains of myosin binding protein C; MD: M-domain of myosin binding protein C containing protein kinase phosphorylation sites.
Similar articles
- Chronic Rho-kinase inhibition improves left ventricular contractile dysfunction in early type-1 diabetes by increasing myosin cross-bridge extension.
Waddingham MT, Edgley AJ, Astolfo A, Inagaki T, Fujii Y, Du CK, Zhan DY, Tsuchimochi H, Yagi N, Kelly DJ, Shirai M, Pearson JT. Waddingham MT, et al. Cardiovasc Diabetol. 2015 Jul 22;14:92. doi: 10.1186/s12933-015-0256-6. Cardiovasc Diabetol. 2015. PMID: 26194354 Free PMC article. - Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity.
Jia G, Hill MA, Sowers JR. Jia G, et al. Circ Res. 2018 Feb 16;122(4):624-638. doi: 10.1161/CIRCRESAHA.117.311586. Circ Res. 2018. PMID: 29449364 Free PMC article. Review. - Pathogenesis of Hypertrophic Cardiomyopathy is Mutation Rather Than Disease Specific: A Comparison of the Cardiac Troponin T E163R and R92Q Mouse Models.
Ferrantini C, Coppini R, Pioner JM, Gentile F, Tosi B, Mazzoni L, Scellini B, Piroddi N, Laurino A, Santini L, Spinelli V, Sacconi L, De Tombe P, Moore R, Tardiff J, Mugelli A, Olivotto I, Cerbai E, Tesi C, Poggesi C. Ferrantini C, et al. J Am Heart Assoc. 2017 Jul 22;6(7):e005407. doi: 10.1161/JAHA.116.005407. J Am Heart Assoc. 2017. PMID: 28735292 Free PMC article. - Functional consequences of sarcomeric protein abnormalities in failing myocardium.
LeWinter MM. LeWinter MM. Heart Fail Rev. 2005 Sep;10(3):249-57. doi: 10.1007/s10741-005-5254-4. Heart Fail Rev. 2005. PMID: 16416047 Review. - The Rho kinase inhibitor, fasudil, ameliorates diabetes-induced cardiac dysfunction by improving calcium clearance and actin remodeling.
Lai D, Gao J, Bi X, He H, Shi X, Weng S, Chen Y, Yang Y, Ye Y, Fu G. Lai D, et al. J Mol Med (Berl). 2017 Feb;95(2):155-165. doi: 10.1007/s00109-016-1469-1. Epub 2016 Aug 30. J Mol Med (Berl). 2017. PMID: 27576917
Cited by
- Diabetes cardiomyopathy: targeted regulation of mitochondrial dysfunction and therapeutic potential of plant secondary metabolites.
Pan X, Hao E, Zhang F, Wei W, Du Z, Yan G, Wang X, Deng J, Hou X. Pan X, et al. Front Pharmacol. 2024 Jul 9;15:1401961. doi: 10.3389/fphar.2024.1401961. eCollection 2024. Front Pharmacol. 2024. PMID: 39045049 Free PMC article. Review. - MicroRNAs Targeting Critical Molecular Pathways in Diabetic Cardiomyopathy Emerging Valuable for Therapy.
Mathur P, Saxena S, Saxena B, Rani V. Mathur P, et al. Cardiovasc Hematol Agents Med Chem. 2024;22(3):298-307. doi: 10.2174/0118715257265947231129074526. Cardiovasc Hematol Agents Med Chem. 2024. PMID: 38265401 Review. - Abnormal left ventricular systolic reserve function detected by treadmill exercise stress echocardiography in asymptomatic type 2 diabetes.
Duan Y, Ye L, Shu Q, Huang Y, Zhang H, Zhang Q, Ding G, Deng Y, Li C, Yin L, Wang Y. Duan Y, et al. Front Cardiovasc Med. 2023 Oct 19;10:1253440. doi: 10.3389/fcvm.2023.1253440. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37928757 Free PMC article. - The interplay of inflammation, exosomes and Ca2+ dynamics in diabetic cardiomyopathy.
Sanganalmath SK, Dubey S, Veeranki S, Narisetty K, Krishnamurthy P. Sanganalmath SK, et al. Cardiovasc Diabetol. 2023 Feb 20;22(1):37. doi: 10.1186/s12933-023-01755-1. Cardiovasc Diabetol. 2023. PMID: 36804872 Free PMC article. Review. - Additive effects of type 2 diabetes and metabolic syndrome on left ventricular torsion and linear deformation abnormalities during dobutamine stress echocardiography.
Aboukhoudir F, Philouze C, Grandperrin A, Nottin S, Obert P. Aboukhoudir F, et al. Front Cardiovasc Med. 2022 Sep 8;9:991415. doi: 10.3389/fcvm.2022.991415. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36158831 Free PMC article.
References
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. - PubMed
- Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. - PubMed
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. Diabetic nephropathy. Diabetes Care. 2003;26 Suppl 1:S94–S98. - PubMed
- Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ. 2014;17:637–645. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources