Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis - PubMed (original) (raw)
Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis
Stig P Cramer et al. Brain. 2015 Sep.
Abstract
Optic neuritis is an acute inflammatory condition that is highly associated with multiple sclerosis. Currently, the best predictor of future development of multiple sclerosis is the number of T2 lesions visualized by magnetic resonance imaging. Previous research has found abnormalities in the permeability of the blood-brain barrier in normal-appearing white matter of patients with multiple sclerosis and here, for the first time, we present a study on the capability of blood-brain barrier permeability in predicting conversion from optic neuritis to multiple sclerosis and a direct comparison with cerebrospinal fluid markers of inflammation, cellular trafficking and blood-brain barrier breakdown. To this end, we applied dynamic contrast-enhanced magnetic resonance imaging at 3 T to measure blood-brain barrier permeability in 39 patients with monosymptomatic optic neuritis, all referred for imaging as part of the diagnostic work-up at time of diagnosis. Eighteen healthy controls were included for comparison. Patients had magnetic resonance imaging and lumbar puncture performed within 4 weeks of onset of optic neuritis. Information on multiple sclerosis conversion was acquired from hospital records 2 years after optic neuritis onset. Logistic regression analysis showed that baseline permeability in normal-appearing white matter significantly improved prediction of multiple sclerosis conversion (according to the 2010 revised McDonald diagnostic criteria) within 2 years compared to T2 lesion count alone. There was no correlation between permeability and T2 lesion count. An increase in permeability in normal-appearing white matter of 0.1 ml/100 g/min increased the risk of multiple sclerosis 8.5 times whereas having more than nine T2 lesions increased the risk 52.6 times. Receiver operating characteristic curve analysis of permeability in normal-appearing white matter gave a cut-off of 0.13 ml/100 g/min, which predicted conversion to multiple sclerosis with a sensitivity of 88% and specificity of 72%. We found a significant correlation between permeability and the leucocyte count in cerebrospinal fluid as well as levels of CXCL10 and MMP9 in the cerebrospinal fluid. These findings suggest that blood-brain barrier permeability, as measured by magnetic resonance imaging, may provide novel pathological information as a marker of neuroinflammation related to multiple sclerosis, to some extent reflecting cellular permeability of the blood-brain barrier, whereas T2 lesion count may more reflect the length of the subclinical pre-relapse phase.See Naismith and Cross (doi:10.1093/brain/awv196) for a scientific commentary on this article.
Keywords: DCE-MRI; blood–brain barrier; multiple sclerosis; optic neuritis; perfusion MRI.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
See Naismith and Cross (doi:10.1093/brain/awv196) for a scientific commentary on this article. Optic neuritis is highly associated with development of multiple sclerosis. Cramer et al. show that MRI measures of blood-brain barrier permeability improve prediction of conversion to multiple sclerosis within 2 years, compared to T2-lesion count alone. Permeability measures also correlate with CSF biomarkers of cellular trafficking and blood-brain barrier breakdown.
Figure 1
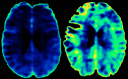
Healthy control subject. (A) T2-weighted sequence on which region of interest placement is performed. Purple: normal-appearing white matter; orange: thalamus; red: lesions. (B) Corresponding DCE slices. (C) Voxel-wise permeability maps, measured as Ki in ml/100 g/min.
Figure 2
Patient with optic neuritis that did not convert to multiple sclerosis. (A) T2-weighted sequence on which region of interest placement is performed. Purple: normal-appearing white matter; orange: thalamus; red: lesions. (B) Corresponding DCE-MRI slices. (C) Voxel-wise permeability maps, measured as Ki in ml/100 g/min.
Figure 3
Optic neuritis patient with conversion to multiple sclerosis. (A) T2-weighted sequence on which region of interest placement is performed. Purple: normal-appearing white matter; Orange: thalamus; Red: lesions. (B) Corresponding DCE slices. (C) Voxel-wise permeability maps, measured as Ki in ml/100 g/min. Note the higher permeability values in normal-appearing white matter, when compared to Fig. 2, which has the same scaling. A small contrast-enhancing lesion that was visible on post-contrast T1 image (not shown) is marked by red arrows. Note that the model fit is more prone to noise-related errors when conducted on a voxel-wise basis compared to the region of interest-based approach used in the article. Also note that the high permeability values in some parts of the ventricular system are caused by the choroid plexus, where fenestrated capillaries and lack of tight junctions allow passage of contrast agent into the interstitial spaces.
Figure 4
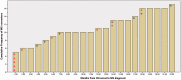
Cumulative incidence of conversion from optic neuritis to multiple sclerosis. The diagram shows the timing of multiple sclerosis (MS) diagnosis and initiation of first-line disease modifying treatment (interferon beta 1a). Note that all patients were untreated at optic neuritis (ON) onset, and decision for treatment initiation was made as either a preventative measure or after multiple sclerosis diagnosis. This decision was made by multiple sclerosis specialist doctors and was not influenced by the study. Red asterisk indicates that patient started on disease-modifying treatment at the same time as multiple sclerosis diagnosis. Blue asterisk indicates that patient started on preventative disease-modifying treatment 2–6 weeks after optic neuritis onset.
Figure 5
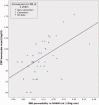
Permeability of the BBB in periventricular normal-appearing white matter plotted against CSF leucocyte count in optic neuritis patients. The blue circle and green star icons indicates multiple sclerosis conversion status for each patient after 2 years. Spearman CC 0.57; P = 0.0002. Linear fit line added for visualization purposes only. No data = no information on multiple sclerosis conversion status. NAWM = normal-appearing white matter.
Figure 6
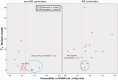
Performance of T2 lesion count, permeability in normal-appearing white matter and CSF leucocytes to predict multiple sclerosis conversion. The vertical dotted line represents the normal-appearing white matter permeability threshold level of 0.13 ml/100 g/min found in the ROC analysis. Four patients (circled with green) were not started on disease-modifying treatment in part due to a low perceived future multiple sclerosis risk. However, all four had normal-appearing white matter permeability >0.13 ml/100 g/min and three had CSF leucocytes >5 mio/l. The blue circle highlights the six false positives that did not develop multiple sclerosis but had permeability >0.13 ml/100 g/min. NAWM = normal-appearing white matter.
Comment in
- Enhancing our understanding of white matter changes in early multiple sclerosis.
Naismith RT, Cross AH. Naismith RT, et al. Brain. 2015 Sep;138(Pt 9):2465-6. doi: 10.1093/brain/awv196. Brain. 2015. PMID: 26304148 No abstract available.
Similar articles
- Risk of multiple sclerosis after optic neuritis in patients with normal baseline brain MRI.
Marques IB, Matias F, Silva ED, Cunha L, Sousa L. Marques IB, et al. J Clin Neurosci. 2014 Apr;21(4):583-6. doi: 10.1016/j.jocn.2013.06.013. Epub 2013 Aug 23. J Clin Neurosci. 2014. PMID: 24231563 - Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: a population-based prospective cohort study.
Olesen MN, Soelberg K, Debrabant B, Nilsson AC, Lillevang ST, Grauslund J, Brandslund I, Madsen JS, Paul F, Smith TJ, Jarius S, Asgari N. Olesen MN, et al. J Neuroinflammation. 2019 Mar 11;16(1):59. doi: 10.1186/s12974-019-1440-5. J Neuroinflammation. 2019. PMID: 30857557 Free PMC article. - Demyelinating optic neuritis in children.
Alper G, Wang L. Alper G, et al. J Child Neurol. 2009 Jan;24(1):45-8. doi: 10.1177/0883073808321052. Epub 2008 Oct 20. J Child Neurol. 2009. PMID: 18936195 Free PMC article. - Oligoclonal bands predict multiple sclerosis after optic neuritis: a literature survey.
Skov AG, Skov T, Frederiksen JL. Skov AG, et al. Mult Scler. 2011 Apr;17(4):404-10. doi: 10.1177/1352458510391340. Epub 2010 Dec 15. Mult Scler. 2011. PMID: 21159718 Review. - Optic neuritis in multiple sclerosis.
Chan JW. Chan JW. Ocul Immunol Inflamm. 2002 Sep;10(3):161-86. doi: 10.1076/ocii.10.3.161.15603. Ocul Immunol Inflamm. 2002. PMID: 12789593 Review.
Cited by
- Measurement variability of blood-brain barrier permeability using dynamic contrast-enhanced magnetic resonance imaging.
Varatharaj A, Jacob C, Darekar A, Yuen B, Cramer S, Larsson H, Galea I. Varatharaj A, et al. Imaging Neurosci (Camb). 2024 Oct 22;2:1-16. doi: 10.1162/imag_a_00324. eCollection 2024 Oct 1. Imaging Neurosci (Camb). 2024. PMID: 39449749 Free PMC article. - Age-related decline in cerebral oxygen consumption in multiple sclerosis.
Knudsen MH, Vestergaard MB, Lindberg U, Simonsen HJ, Frederiksen JL, Cramer SP, Larsson HB. Knudsen MH, et al. J Cereb Blood Flow Metab. 2024 Jun;44(6):1039-1052. doi: 10.1177/0271678X231224502. Epub 2024 Jan 8. J Cereb Blood Flow Metab. 2024. PMID: 38190981 - Understanding the Pathophysiology and Magnetic Resonance Imaging of Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders.
Cacciaguerra L, Rocca MA, Filippi M. Cacciaguerra L, et al. Korean J Radiol. 2023 Dec;24(12):1260-1283. doi: 10.3348/kjr.2023.0360. Korean J Radiol. 2023. PMID: 38016685 Free PMC article. Review. - Editorial: Common pathogenic mechanism of cerebrovascular disease and degenerative diseases.
Zheng W, Liang Y, Fan D, He J. Zheng W, et al. Front Neurosci. 2023 Jun 23;17:1233204. doi: 10.3389/fnins.2023.1233204. eCollection 2023. Front Neurosci. 2023. PMID: 37425002 Free PMC article. No abstract available. - Direct association with the vascular basement membrane is a frequent feature of myelinating oligodendrocytes in the neocortex.
Palhol JSC, Balia M, Sánchez-Román Terán F, Labarchède M, Gontier E, Battefeld A. Palhol JSC, et al. Fluids Barriers CNS. 2023 Apr 3;20(1):24. doi: 10.1186/s12987-023-00425-4. Fluids Barriers CNS. 2023. PMID: 37013659 Free PMC article.
References
- Avolio C, Ruggieri M, Giuliani F, Liuzzi GM, Leante R, Riccio P, et al. Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J Neuroimmunol 2003; 136: 46–53. - PubMed
- Barkhof F, Miller D, Scheltens P, Campi A, Polman C, Filippi M. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120: 2059–69. - PubMed
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol 2007; 28: 5–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous