γ-Tilmanocept, a New Radiopharmaceutical Tracer for Cancer Sentinel Lymph Nodes, Binds to the Mannose Receptor (CD206) - PubMed (original) (raw)
γ-Tilmanocept, a New Radiopharmaceutical Tracer for Cancer Sentinel Lymph Nodes, Binds to the Mannose Receptor (CD206)
Abul K Azad et al. J Immunol. 2015.
Abstract
γ-Tilmanocept ((99m)Tc-labeled-tilmanocept or [(99m)Tc]-tilmanocept) is the first mannose-containing, receptor-directed, radiolabeled tracer for the highly sensitive imaging of sentinel lymph nodes in solid tumor staging. To elucidate the mannose-binding receptor that retains tilmanocept in this microenvironment, human macrophages were used that have high expression of the C-type lectin mannose receptor (MR; CD206). Cy3-labeled tilmanocept exhibited high specificity binding to macrophages that was nearly abolished in competitive inhibition experiments. Furthermore, Cy3-tilmanocept binding was markedly reduced on macrophages deficient in the MR by small interfering RNA treatment and was increased on MR-transfected HEK 293 cells. Finally, confocal microscopy revealed colocalization of Cy3-tilmanocept with the macrophage membrane MR and binding of labeled tilmanocept to MR(+) cells (macrophages and/or dendritic cells) in human sentinel lymph node tissues. Together these data provide strong evidence that CD206 is a major binding receptor for γ-tilmanocept. Identification of CD206 as the γ-tilmanocept-binding receptor enables opportunities for designing receptor-targeted advanced imaging agents and therapeutics for cancer and other diseases.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures
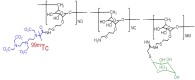
FIGURE 1.
[99mTc]-(γ)-tilmanocept chemical structure.
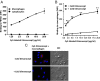
FIGURE 2.
Tilmanocept binds to human macrophages and not lymphocytes. Five-day-old PBMCs (consisting of lymphocytes and MDMs) were incubated with increasing concentrations (1.25, 2.5, 5.0, 10, and 20 μg/ml) of Cy3-tilmanocept in the presence or absence of excess unlabeled tilmanocept. Tilmanocept binding to cells was then evaluated by either flow cytometry or fluorescence confocal microscopy. (A) Flow-cytometric analysis of PBMCs incubated with increasing concentrations of Cy3-tilmanocept and gated for macrophages or lymphocytes. (B) PBMCs were incubated with increasing concentrations of Cy3-tilmanocept in the presence or absence of 100-fold excess unlabeled (cold) tilmanocept. Shown is flow-cytometric analysis of macrophages showing inhibition of Cy3-tilmanocept binding to macrophages in presence of cold tilmanocept. **p < 0.005. (C) Representative confocal microscopic images (original magnification ×1200) showing binding (upper left panel) and inhibition of binding (lower left panel) of Cy3-tilmanocept to macrophages in absence or presence of unlabeled tilmanocept, respectively. Blue represents DAPI staining of macrophage nuclei; red represents Cy3-tilmanocept. Data are representative of two independent experiments, each performed in duplicate. DIC, differential interference contrast.
FIGURE 3.
Binding of tilmanocept to human macrophages is nearly abolished after treatment with mannan. MDMs were incubated without or with mannan or GalNAc followed by incubation with Cy3-tilmanocept. Tilmanocept binding was evaluated by confocal microscopy. (A) Representative confocal images (original magnification ×1200) showing binding of Cy3-tilmanocept to mannan-untreated macrophages (upper left panel) and inhibition of its binding after mannan pretreatment (lower left panel). (B) Bar graph showing the difference in MFI A.U. for tilmanocept binding between untreated and mannan-pretreated macrophages. (C) Representative confocal images (original magnification ×1200) showing binding of Cy3-tilmanocept to GalNAc-untreated macrophages (upper left panel) and after GalNAC pretreatment (lower left panel). (D) Bar graph showing mean MFI values for tilmanocept binding between untreated and GalNAc-pretreated macrophages. Blue represents DAPI staining of macrophage nuclei; red represents Cy3-tilmanocept. The MFI was determined for ∼350–400 macrophages per coverslip, with duplicate slides per experiment of at least two independent experiments of each category (**p < 0.005). DIC, differential interference contrast.
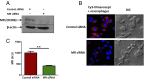
FIGURE 4.
Reduced binding of tilmanocept to MR-deficient human macrophages. MDMs were transfected with MR-siRNA or scramble control siRNA by nucleofection. Treated MDMs were then incubated with Cy3-tilmanocept, and the binding was analyzed by confocal microscopy. (A) Western blot showing siRNA-mediated knockdown of the MR protein; β-actin was used as the loading control. (B) Representative confocal images (original magnification ×1200) showing binding of tilmanocept to control macrophages (upper left panel) and markedly reduced binding of tilmanocept to MR-deficient macrophages (lower left panel). Blue represents DAPI staining of macrophage nuclei; red represents Cy3-tilmanocept. (C) Bar graph showing the difference in MFI (A.U.) between the control and MR-deficient macrophages. The MFI was determined for ∼150 macrophage cells per coverslip, using duplicate slides per experiment (n = 2; **p < 0.005). DIC, differential interference contrast.
FIGURE 5.
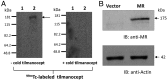
Binding of tilmanocept to the MR protein by lectin blot. HEK293 cells, transfected with empty vector or an MR expression plasmid, were lysed, and total proteins (20 μg from each sample) were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membrane was preincubated with or without 100-fold excess cold tilmanocept, followed by incubation with [99mTc]-tilmanocept (γ-tilmanocept, 20 μg/ml) for 2 h, washed and exposed to autoradiography. (A) Autoradiograms showing binding of [99mTc]-tilmanocept to the MR protein (arrow) on an untreated blot (left panel) and lack of [99mTc]-tilmanocept binding to a blot that was pretreated with cold tilmanocept (right panel). (A and B) Lane 1, cell lysate of HEK293 cells transfected with empty vector; lane 2, cell lysate of HEK293 cells transfected with the MR expression plasmid. Molecular weight markers are shown on the left of each blot. (B) Western blot showing expression of the MR protein in transfected HEK293 cells (upper panel). The same blot was reprobed with anti-actin Ab to show β-actin as an internal protein control (lower panel). Results shown are representative of two independent experiments.
FIGURE 6.
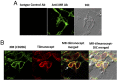
MR expression and colocalization with tilmanocept on human macrophages. (A) MDM monolayers were fixed with paraformaldehyde (without permeabilization), incubated with either the isotype control Ab or the anti-MR Ab, and stained with AF488-conjugated secondary Ab. The monolayers were then analyzed by confocal microscopy. Representative confocal images (original magnification ×1200) showing MR expression (green in middle panel) and no staining in the case of the isotype control Ab (left panel). Right panel shows differential interference contrast (DIC; phase-contrast microscopy). Data are representative of n = 2 in duplicate. (B) MDM monolayers were incubated with Cy3-tilmanocept for 10 min, fixed with paraformaldehyde, incubated with anti-MR Ab, and stained with AF488-conjugated secondary Ab. The monolayers were then analyzed by confocal microscopy. Representative confocal images (original magnification ×1600) showing MR expression (green in first panel), tilmanocept binding by the macrophage (red in second panel), and colocalization of the MR and tilmanocept in both confocal and phase-contrast images (yellow in third and fourth panels, arrow shows an example of intense colocalization). Results shown are representative of three independent experiments.
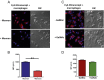
FIGURE 7.
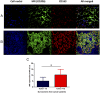
Expression of the MR (CD206) and CD163 by macrophages present in tumor-negative and tumor-positive lymph nodes from cancer patients. FFPE tissue sections from tumor-negative (A) and tumor-positive (B) lymph nodes from cancer patients were deparaffinized, followed by an Ag retrieval procedure. Sections were then subjected to double staining with Abs against the MR and CD163, and analyzed by confocal microscopy. Representative confocal images (original magnification ×400) showing the total number of cells (blue, nuclear staining) and expression of the MR (green) and CD163 (red) in tissue sections from tumor-negative (A) and tumor-positive (B) lymph nodes from cancer patients. Results shown are representative of 3 independent experiments using a total of 12 tissue sections (4 sections from tumor-positive and tumor-negative SLNs from each patient, n = 3 patients). (C) MR expression was quantified in 20 randomly selected confocal images using the NIH ImageJ program, 10 each from tumor-negative and tumor-positive tissue sections (*p < 0.05). The MFI in A.U. shown represents an average value (± SD) per image section.
FIGURE 8.
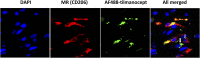
Binding of tilmanocept to MR+ cells in SLN tissues. FFPE tissue sections were subjected to deparaffinization and Ag retrieval procedure followed by incubation with AF488-labeled tilmanocept for 24 h. Sections were then stained with DAPI and anti-MR Ab, and analyzed by confocal microscopy. Representative confocal images (original magnification ×1600) showing the total number of cells (blue represents nuclear staining by DAPI in first panel), MR+ cells (red in second panel), and binding of tilmanocept (green in third panel) to these cells, with colocalization (yellow in fourth panel). Solid white arrow indicates tilmanocept binding to a non–MR-expressing cell; open arrow indicates no binding to an MR-expressing cell. Results shown are representative of a total of 18 tissue sections from 6 patients.
FIGURE 9.
Lack of MR expression and tilmanocept binding by LYVE-1+ endothelial cells in SLN tissues. FFPE tissue sections were processed for Ag retrieval (see immunohistochemistry method) and then stained with DAPI, anti–LYVE-1, and anti-MR Abs (A and C) or incubated with AF488-labeled tilmanocept followed by staining with DAPI and anti–LYVE-1 Ab (B and D). Sections were analyzed by confocal microscopy. Representative confocal images (original magnification ×800) show LYVE-1+ endothelial cells (red) in vascular-appearing (A and B) and other (C and D) regions of the SLN tissues. MR expression (A and C) and tilmanocept binding (B and D) by lymph node cells are shown in green. Arrow indicates an example of MR expression (A) or tilmanocept binding (B) by LYVE-1− cells outside the LYVE-1+ lymphatic endothelium. Blue indicates nuclear staining by DAPI. Results shown are representative of three independent experiments with a total of six tissue sections from three patients.
Similar articles
- Cy3-tilmanocept labeling of macrophages in joints of mice with antibody-induced arthritis and synovium of human patients with rheumatoid arthritis.
Toribio RE, Young N, Schlesinger LS, Cope FO, Ralph DA, Jarjour W, Rosol TJ. Toribio RE, et al. J Orthop Res. 2021 Apr;39(4):821-830. doi: 10.1002/jor.24900. Epub 2020 Dec 15. J Orthop Res. 2021. PMID: 33107629 - Detection of melanoma, breast cancer and head and neck squamous cell cancer sentinel lymph nodes by Tc-99m Tilmanocept (Lymphoseek®).
Leong SP. Leong SP. Clin Exp Metastasis. 2022 Feb;39(1):39-50. doi: 10.1007/s10585-021-10137-4. Epub 2021 Dec 28. Clin Exp Metastasis. 2022. PMID: 34962630 Free PMC article. Review. - Molecular Imaging of the Glomerulus via Mesangial Cell Uptake of Radiolabeled Tilmanocept.
Qin Z, Hoh CK, Olson ES, Jahromi AH, Hall DJ, Barback CV, You YH, Yanagita M, Sharma K, Vera DR. Qin Z, et al. J Nucl Med. 2019 Sep;60(9):1325-1332. doi: 10.2967/jnumed.118.223727. Epub 2019 Feb 22. J Nucl Med. 2019. PMID: 30796169 Free PMC article. - [99mTc]Tilmanocept Lymphscintigraphy after inconclusive [99mTc]Nanocolloid Scan in Breast Cancer.
Kircher M, Seifert S, Kircher S, Lapa C. Kircher M, et al. Nuklearmedizin. 2019 Jun;58(3):282-284. doi: 10.1055/a-0891-7686. Epub 2019 May 7. Nuklearmedizin. 2019. PMID: 31064028 - 99mTc-Tilmanocept: A Novel Molecular Agent for Lymphatic Mapping and Sentinel Lymph Node Localization.
Surasi DS, O'Malley J, Bhambhvani P. Surasi DS, et al. J Nucl Med Technol. 2015 Jun;43(2):87-91. doi: 10.2967/jnmt.115.155960. Epub 2015 May 8. J Nucl Med Technol. 2015. PMID: 25956693 Review.
Cited by
- Carbohydrate-based drugs launched during 2000_-_2021.
Cao X, Du X, Jiao H, An Q, Chen R, Fang P, Wang J, Yu B. Cao X, et al. Acta Pharm Sin B. 2022 Oct;12(10):3783-3821. doi: 10.1016/j.apsb.2022.05.020. Epub 2022 May 23. Acta Pharm Sin B. 2022. PMID: 36213536 Free PMC article. Review. - Tumor-Associated Macrophages-Implications for Molecular Oncology and Imaging.
Kimm MA, Klenk C, Alunni-Fabbroni M, Kästle S, Stechele M, Ricke J, Eisenblätter M, Wildgruber M. Kimm MA, et al. Biomedicines. 2021 Apr 2;9(4):374. doi: 10.3390/biomedicines9040374. Biomedicines. 2021. PMID: 33918295 Free PMC article. Review. - Optical Molecular Imaging of Inflammatory Cells in Interventional Medicine-An Emerging Strategy.
Birch GP, Campbell T, Bradley M, Dhaliwal K. Birch GP, et al. Front Oncol. 2019 Sep 12;9:882. doi: 10.3389/fonc.2019.00882. eCollection 2019. Front Oncol. 2019. PMID: 31572676 Free PMC article. Review. - Identification of CD206 as a potential biomarker of cancer stem-like cells and therapeutic agent in liver cancer.
Fan W, Yang X, Huang F, Tong X, Zhu L, Wang S. Fan W, et al. Oncol Lett. 2019 Sep;18(3):3218-3226. doi: 10.3892/ol.2019.10673. Epub 2019 Jul 26. Oncol Lett. 2019. PMID: 31452799 Free PMC article. - Targeting Tumors Using Peptides.
Scodeller P, Asciutto EK. Scodeller P, et al. Molecules. 2020 Feb 13;25(4):808. doi: 10.3390/molecules25040808. Molecules. 2020. PMID: 32069856 Free PMC article. Review.
References
- Vera D. R., Wallace A. M., Hoh C. K. 2001. [(99m)Tc]MAG(3)-mannosyl-dextran: a receptor-binding radiopharmaceutical for sentinel node detection. Nucl. Med. Biol. 28: 493–498. - PubMed
- Hoh C. K., Wallace A. M., Vera D. R. 2003. Preclinical studies of [(99m)Tc]DTPA-mannosyl-dextran. Nucl. Med. Biol. 30: 457–464. - PubMed
- Vera D. R., Wallace A. M., Hoh C. K., Mattrey R. F. 2001. A synthetic macromolecule for sentinel node detection: (99m)Tc-DTPA-mannosyl-dextran. J. Nucl. Med. 42: 951–959. - PubMed
- Méndez J., Wallace A. M., Hoh C. K., Vera D. R. 2003. Detection of gastric and colonic sentinel nodes through endoscopic administration of 99mTc-DTPA-mannosyl-dextran in pigs. J. Nucl. Med. 44: 1677–1681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous