Intrinsic carnosine metabolism in the human kidney - PubMed (original) (raw)
Intrinsic carnosine metabolism in the human kidney
Verena Peters et al. Amino Acids. 2015 Dec.
Abstract
Histidine-containing dipeptides like carnosine and anserine have protective functions in both health and disease. Animal studies suggest that carnosine can be metabolized within the kidney. The goal of this study was to obtain evidence of carnosine metabolism in the human kidney and to provide insight with regards to diabetic nephropathy. Expression, distribution, and localization of carnosinase-1 (CNDP1), carnosine synthase (CARNS), and taurine transporters (TauT) were measured in human kidneys. CNDP1 and CARNS activities were measured in vitro. CNDP1 and CARNS were located primarily in distal and proximal tubules, respectively. Specifically, CNDP1 levels were high in tubular cells and podocytes (20.3 ± 3.4 and 15 ± 3.2 ng/mg, respectively) and considerably lower in endothelial cells (0.5 ± 0.1 ng/mg). CNDP1 expression was correlated with the degradation of carnosine and anserine (r = 0.88 and 0.81, respectively). Anserine and carnosine were also detectable by HPLC in the renal cortex. Finally, TauT mRNA and protein were found in all renal epithelial cells. In diabetic patients, CNDP1 seemed to be reallocated to proximal tubules. We report compelling evidence that the kidney has an intrinsic capacity to metabolize carnosine. Both CNDP1 and CARNS are expressed in glomeruli and tubular cells. Carnosine-synthesizing and carnosine-hydrolyzing enzymes are localized in distinct compartments in the nephron and increased CNDP1 levels suggest a higher CNDP1 activity in diabetic kidneys.
Keywords: Anserine; Carnosinase (CNDP1); Carnosine; Diabetic nephropathy; Metabolism.
Figures
Fig. 1
a Immunohistochemistry showing the presence of CNDP1 in the glomeruli and distal tubules (arrows). b Immunohistochemistry showing CARNS expression in proximal tubules and glomeruli (arrows). The nuclei were counterstained with hematoxylin. c Immunofluorescence showing carnosinase (red) and carnosine synthase (green) in separate compartments in tubular cells. Immunohistochemistry showing CARNS expression in proximal tubules and glomeruli (arrows). d Immunofluorescence showing non-overlapping expression of CARNS (red) in the proximal tubules and Tamm-Horsfall protein (green) in the distal tubules. e Immunohistochemistry showing that the TauT is expressed in proximal tubules (red arrow) and distal tubules (black arrow) in human kidney samples. The highest proteins levels were present in the distal tubules. The nuclei were counterstained with hematoxylin
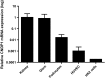
Fig. 2
CDNP1 mRNA was amplified from human kidney samples (1.00 ± 1.12) (N = 8), human glomeruli (Glom) (0.802 ± 1.1) (N = 8), immortalized human podocytes (0.016 ± 0.0013) (N = 2), human endothelial cells (HUVEC) (0.001 ± 0.0013) (N = 5), and human proximal tubular epithelial cells (HK2 cells) (0.000185) (N = 1). All values were normalized to the mean value obtained from the human kidney samples. Note that the _y_-axis is plotted on a logarithmic scale, expressed as mean ± SD of relative units
Fig. 3
CNDP1 activity (in nmol/mg/h) was measured as the rate of degradation of carnosine (black) and anserine (grey) in mouse podocytes (2.8 ± 1.7 and 2.9 ± 1.5 nmol/mg/h, respectively) and human proximal tubular epithelial cells (HK2 cells) (1.3 ± 0.4 and 0.05 ± 0.08 nmol/mg/h, respectively), expressed as mean ± SD
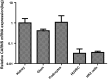
Fig. 4
CARNS mRNA was amplified from human kidney samples (1.00 ± 0.63) (N = 8), glomeruli (Glom) (0.4136 ± 0.08) (N = 4), immortalized human podocytes (1.07 ± 0.15) (N = 2), human endothelial cells (HUVEC) (0.032 ± 0.02) (N = 5), and proximal tubular epithelial cells (HK2 cells) (0.035 ± 0.005) (N = 2). All values were normalized to the mean value obtained from the human kidney samples. Note that the _y_-axis is plotted on a logarithmic scale, expressed as mean ± SD of relative units
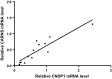
Fig. 5
Relative CARNS mRNA level is plotted against the relative CNDP1 mRNA level measured in human kidney samples. Each data point represents a separate sample. The solid line is a linear fit of the data. r = 0.81 (N = 13)
Fig. 6
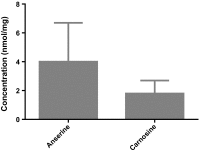
Concentrations of anserine (4 ± 2.7) [95 % CI 2.3–6.4] and carnosine (1.8 ± 0.9) [95 % CI 1.1–2.8] in human kidney samples (N = 3) measured using HPLC, expressed as mean ± SD
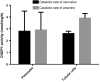
Fig. 7
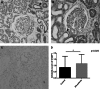
CNDP1 in diabetic patient (N = 14) and control (N = 7). Immunohistochemistry and intensity score *(p = 0.029). It shows a reallocation of CNDP1 from distal to proximal tubules in diabetic patients with renal damage. a Healthy control (0.857 ± 0.8997), b diabetic nephropathy patients (1.171 ± 0.726), c negative control, d intensity staining difference; red arrow proximal tubules, black arrow distal tubules; expressed in mean ± SD
Similar articles
- A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice.
Weigand T, Colbatzky F, Pfeffer T, Garbade SF, Klingbeil K, Colbatzky F, Becker M, Zemva J, Bulkescher R, Schürfeld R, Thiel C, Volk N, Reuss D, Hoffmann GF, Freichel M, Hecker M, Poth T, Fleming T, Poschet G, Schmitt CP, Peters V. Weigand T, et al. Int J Mol Sci. 2020 Jul 10;21(14):4887. doi: 10.3390/ijms21144887. Int J Mol Sci. 2020. PMID: 32664451 Free PMC article. - Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration.
Peters V, Jansen EE, Jakobs C, Riedl E, Janssen B, Yard BA, Wedel J, Hoffmann GF, Zschocke J, Gotthardt D, Fischer C, Köppel H. Peters V, et al. Clin Chim Acta. 2011 Jan 30;412(3-4):263-7. doi: 10.1016/j.cca.2010.10.016. Epub 2010 Oct 21. Clin Chim Acta. 2011. PMID: 20971102 - Carnosine Catalyzes the Formation of the Oligo/Polymeric Products of Methylglyoxal.
Weigand T, Singler B, Fleming T, Nawroth P, Klika KD, Thiel C, Baelde H, Garbade SF, Wagner AH, Hecker M, Yard BA, Amberger A, Zschocke J, Schmitt CP, Peters V. Weigand T, et al. Cell Physiol Biochem. 2018;46(2):713-726. doi: 10.1159/000488727. Epub 2018 Mar 29. Cell Physiol Biochem. 2018. PMID: 29621776 - [Carnosine, carnosinase and kidney diseases].
Kiliś-Pstrusińska K. Kiliś-Pstrusińska K. Postepy Hig Med Dosw (Online). 2012 Apr 20;66:215-21. doi: 10.5604/17322693.991600. Postepy Hig Med Dosw (Online). 2012. PMID: 22706107 Review. Polish. - Carnosine and Diabetic Nephropathy.
Peters V, Yard B, Schmitt CP. Peters V, et al. Curr Med Chem. 2020;27(11):1801-1812. doi: 10.2174/0929867326666190326111851. Curr Med Chem. 2020. PMID: 30914013 Review.
Cited by
- A capillary electrophoresis coupled to mass spectrometry pipeline for long term comparable assessment of the urinary metabolome.
Boizard F, Brunchault V, Moulos P, Breuil B, Klein J, Lounis N, Caubet C, Tellier S, Bascands JL, Decramer S, Schanstra JP, Buffin-Meyer B. Boizard F, et al. Sci Rep. 2016 Oct 3;6:34453. doi: 10.1038/srep34453. Sci Rep. 2016. PMID: 27694997 Free PMC article. Clinical Trial. - Metabolomics approach reveals urine biomarkers and pathways associated with the pathogenesis of lupus nephritis.
Kalantari S, Chashmniam S, Nafar M, Zakeri Z, Parvin M. Kalantari S, et al. Iran J Basic Med Sci. 2019 Nov;22(11):1288-1295. doi: 10.22038/ijbms.2019.38713.9178. Iran J Basic Med Sci. 2019. PMID: 32128093 Free PMC article. - Carnosine Attenuates the Development of both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice.
Albrecht T, Schilperoort M, Zhang S, Braun JD, Qiu J, Rodriguez A, Pastene DO, Krämer BK, Köppel H, Baelde H, de Heer E, Anna Altomare A, Regazzoni L, Denisi A, Aldini G, van den Born J, Yard BA, Hauske SJ. Albrecht T, et al. Sci Rep. 2017 Mar 10;7:44492. doi: 10.1038/srep44492. Sci Rep. 2017. PMID: 28281693 Free PMC article. - Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress.
Menini S, Iacobini C, Vitale M, Pesce C, Pugliese G. Menini S, et al. Cancers (Basel). 2021 Jan 16;13(2):313. doi: 10.3390/cancers13020313. Cancers (Basel). 2021. PMID: 33467038 Free PMC article. Review. - A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice.
Weigand T, Colbatzky F, Pfeffer T, Garbade SF, Klingbeil K, Colbatzky F, Becker M, Zemva J, Bulkescher R, Schürfeld R, Thiel C, Volk N, Reuss D, Hoffmann GF, Freichel M, Hecker M, Poth T, Fleming T, Poschet G, Schmitt CP, Peters V. Weigand T, et al. Int J Mol Sci. 2020 Jul 10;21(14):4887. doi: 10.3390/ijms21144887. Int J Mol Sci. 2020. PMID: 32664451 Free PMC article.
References
- Adelmann K, Frey D, Riedl E, Koeppel H, Pfister F, Peters V, Schmitt CP, Sternik P, Hofmann S, Zentgraf HW, Navis G, van den Born J, Bakker SJ, Kramer BK, Yard BA, Hauske SJ. Different conformational forms of serum carnosinase detected by a newly developed sandwich ELISA for the measurements of carnosinase concentrations. Amino Acid. 2012;43:143–151. doi: 10.1007/s00726-012-1244-8. - DOI - PubMed
- Alhamdani MS, Al-Azzawie HF, Abbas FK. Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit Dial Int. 2007;27:86–89. - PubMed
- Babizhayev MA, Lankin VZ, Savel’Yeva EL, Deyev AI, Yegorov YE. Diabetes mellitus: novel insights, analysis and interpretation of pathophysiology and complications management with imidazole-containing peptidomimetic antioxidants. Recent Pat Drug Deliv Formul. 2013;7:216–256. doi: 10.2174/1872211307666131117121058. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous