Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress - PubMed (original) (raw)
Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress
William M Oldham et al. Cell Metab. 2015.
Abstract
Metabolic adaptation to hypoxia is critical for survival in metazoan species for which reason they have developed cellular mechanisms for mitigating its adverse consequences. Here, we have identified L-2-hydroxyglutarate (L2HG) as a universal adaptive determinant of the hypoxia response. L2HG is a metabolite of unknown function produced by the reduction of mitochondrial 2-oxoglutarate by malate dehydrogenase. L2HG accumulates in response to increases in 2-oxoglutarate, which occur as a result of tricarboxylic acid cycle dysfunction and increased mitochondrial reducing potential. These changes are closely coupled to cellular redox homeostasis, as increased cellular L2HG inhibits electron transport and glycolysis to offset the adverse consequences of mitochondrial reductive stress induced by hypoxia. Thus, L2HG couples mitochondrial and cytoplasmic energy metabolism in a model of cellular redox regulation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
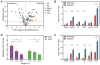
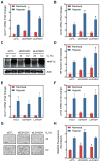
Figure 1. Hypoxia Increases 2OG and 2HG
(A) Volcano plot of metabolite changes in PASMC treated with 0.2% oxygen for 24 h compared to 21% oxygen controls. AA, amino acids; NA, nucleic acid pathway intermediates; PPP, pentose phosphate pathway intermediates; TCA, tricarboxylic acid cycle intermediates. (B and C) Targeted LCMS reveals that hypoxia-mediated increases in 2OG (B) and 2HG (C) occur in a variety of cell types. PAEC, human pulmonary artery endothelial cells; PASMC, human pulmonary artery smooth muscle cells; LF, human lung fibroblasts; HEK, human embryonic kidney 293 cells; HepG2, human liver hepatocellular carcinoma cells; MSC, human mesenchymal stem cells. (D) Intracellular 2OG and 2HG vary inversely with oxygen tension in PASMC (p < 0.0001 for trend for each). Data are mean ± SEM. See also Figure S1.
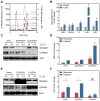
Figure 2. Hypoxia Preferentially Increases the L Entantiomer of 2HG
(A) Extracted ion chromatograms (XIC) of underivatized 2HG (purple) and derivatized enantiomerically pure standards of D2HG (green), L2HG (blue), or a racemic mixture (red). Chromatograms are offset for clarity. The XIC of a derivatized cell extract is also shown (black) with intensity values on the right _y_-axis. (B) Fold change after hypoxia treatment of D2HG and L2HG in derivatized cell extracts. (C) Immunoblot of D2HGDH and L2HGDH levels in LF treated with targeting siRNA compared to a non-targeting control siRNA (siCTL). (D) Total 2HG in cell extracts treated with D2HGDH or L2HGDH siRNA. (E) Immunoblot of LF cell extracts overexpressing D2HGDH or L2HGDH. (F) Total 2HG in LF overexpressing D2HGDH or L2HGDH compared to Vector control. Data are mean ± SEM. See also Figure S2.
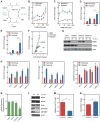
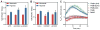
Figure 3. Potential Mechanisms of L2HG Accumulation in Hypoxia
(A) Enzymatic and cofactor requirements for L2HG metabolism. (B and C) Intracellular 2OG (B) and 2HG (C) in normoxia and hypoxia measured after treatment with a cell-permeable 2OG analogue, TFMB-2OG. (D and E) 2OG (D) and 2HG (E) in cells treated with the OGDHC inhibitor KMV (20 mM) ± 0.2% oxygen. (F) Correlation between 2OG and 2HG from experiments illustrated in B-E. (G) Immunoblot of MDH1 and MDH2 in siRNA-treated LF. (H–J) 2OG (H), 2HG (I), and 2OG/2HG (J) in MDH knockdown cells. (K) Relative changes in mRNA levels of 2HG metabolic enzymes in response to hypoxia. (L) Immunoblot of LF cell lysates of proteins involved in L2HG metabolism. (M) Intracellular malate determined by targeted LC-MS. (M) Cellular NADH/NAD+ determined by enzymatic cycling assay. Data are mean ± SEM. See also Figure S3.
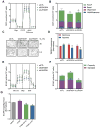
Figure 4. Role of HIF in 2HG Metabolism
(A) Immunoblot of LF treated with siRNA targeting VHL mRNA compared to siCTL demonstrating stabilization of HIF1α and HIF2α in normoxia. (B) Normoxic stabilization of HIF1α and HIF2α increases GLUT1 and SERPINE1 mRNA, but has no effect on L2HGDH mRNA. (C) HIF stabilization through VHL knockdown is sufficient to increase 2HG and 2OG in normoxia. (D) Immunoblot demonstrating effective knockdown of HIF1α and HIF2α in normoxia and hypoxia. (E) Silencing HIF1α blunts the hypoxia-mediated increase in GLUT1 expression. (F) Silencing HIF2α blunts the hypoxia-mediated increase in SERPINE1 expression. (G and H) 2HG and 2OG in cells treated with HIF siRNA in normoxia and hypoxia. (I) Levels of L2HGDH mRNA in cells treated with siRNA targeting hypoxia-regulated transcription factors. Data are mean ± SEM. See also Figure S4.
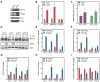
Figure 5. D2HGDH and L2HGDH Knockdown Activate HIF Target Gene Expression
(A–B, E–F) Expression of HIF target genes, GLUT1 (A and E) and LDHA (B and F) in LF treated with siD2HGDH and siL2HGDH (A and B) or D2HGDH and L2HGDH (E and F). (C) Representative immunoblot demonstrating normoxic HIF1α stabilization by siD2HGDH and siL2HGDH. (D) Quantification of HIF1α densitometry. (G) Representative micrographs of MitoSOX-stained cells. Images were inverted and leveled for presentation. Scale bar is 20 μm. (H) Quantification of MitoSOX staining. Data are mean ± SEM. See also Figure S5.
Figure 6. 2HG Metabolism Is Coupled to Cellular Redox State
(A and B) NADH/NAD+ ratio determined by enzymatic cycling assay in LF treated with siRNA targeting D2HGDH or L2HGDH expression (A) or overexpressing D2HGDH or L2HGDH (B). (C) F420/F485 fluorescence ratio, corresponding to cytoplasmic NADH/NAD+, in LF treated with permeable analogues of 2OG, D2HG, and L2HG (500 μM) at time 0 normalized to the fluorescence ratio of untreated cells. Data are mean ± SEM. See also Figure S6.
Figure 7. D2HGDH and L2HGDH Silencing Affects Oxidative Phosphorylation and Glycolysis
(A) Oxygen consumption rate (OCR) of LF treated with siRNA targeting D2HGDH or L2HGDH after treatment with the indicated compounds (Oligo, oligomycin; AMA, antimycin A). (B) Summarized data from (A). (C) Representative micrographs of TMRM-stained LF. Images were inverted and leveled for presentation. Scale bar is 20 μm. Scale bar is 20 μm. (D) Quantification of TMRM staining in normoxia and hypoxia in siRNA-treated LF. (E) Extracellular acidification rate (ECAR) of LF treated with siRNA targeting D2HGDH or L2HGDH before and after treatment with oligomycin to measure glycolytic capacity (2DG, 2-deoxyglucose). (F) Summarized data from (E). (G) Cell-permeable analogues of D2HG and L2HG inhibit oligomycin-stimulated ECAR. Data are mean ± SEM. See also Figure S7.
References
- Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–741. - PubMed
- Chalmers RA, Lawson AM, Watts RW, Tavill AS, Kamerling JP, Hey E, Ogilvie D. D-2-hydroxyglutaric aciduria: case report and biochemical studies. J Inherit Metab Dis. 1980;3:11–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL108630/HL/NHLBI NIH HHS/United States
- HL007633/HL/NHLBI NIH HHS/United States
- T32 HL007633/HL/NHLBI NIH HHS/United States
- P01 HL048743/HL/NHLBI NIH HHS/United States
- HL048743/HL/NHLBI NIH HHS/United States
- R01 HL061795/HL/NHLBI NIH HHS/United States
- P50 GM107618/GM/NIGMS NIH HHS/United States
- HL108630/HL/NHLBI NIH HHS/United States
- HL061795/HL/NHLBI NIH HHS/United States
- R37 HL061795/HL/NHLBI NIH HHS/United States
- GM107618/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources