Morphogenesis of the Fission Yeast Cell through Cell Wall Expansion - PubMed (original) (raw)
Morphogenesis of the Fission Yeast Cell through Cell Wall Expansion
Erdinc Atilgan et al. Curr Biol. 2015.
Abstract
The shape of walled cells such as fungi, bacteria, and plants are determined by the cell wall. Models for cell morphogenesis postulate that the effects of turgor pressure and mechanical properties of the cell wall can explain the shapes of these diverse cell types. However, in general, these models await validation through quantitative experiments. Fission yeast Schizosaccharomyces pombe are rod-shaped cells that grow by tip extension and then divide medially through formation of a cell wall septum. Upon cell separation after cytokinesis, the new cell ends adopt a rounded morphology. Here, we show that this shape is generated by a very simple mechanical-based mechanism in which turgor pressure inflates the elastic cell wall in the absence of cell growth. This process is independent of actin and new cell wall synthesis. To model this morphological change, we first estimate the mechanical properties of the cell wall using several approaches. The lateral cell wall behaves as an isotropic elastic material with a Young's modulus of 50 ± 10 MPa inflated by a turgor pressure estimated to be 1.5 ± 0.2 MPa. Based upon these parameters, we develop a quantitative mechanical-based model for new end formation that reveals that the cell wall at the new end expands into its characteristic rounded shape in part because it is softer than the mature lateral wall. These studies provide a simple example of how turgor pressure expands the elastic cell wall to generate a particular cell shape.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
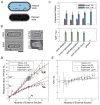
Figure 1. Shaping of the new end cell wall and septum are independent of actin and wall synthesis
A) During cell-cell separation, the process starts with a sudden break in the lateral cell wall (−1 to 0 s) followed by formation of a rounded new cell end (0–15 m). Movie 1. The plot shows bulging of the NE wall (as measured by the “s value”) over time. B) Deformation of the septum. In a cell with a complete septum, one of the cellular compartments was lysed by a laser cut to the cell surface (yellow asterisk). The septum forms a curved shape in ≤ 10 ms (within a single frame, arrowhead. See Movie 2.) C) Septum deformation is actin independent. Similar as in B, except one compartment of cells (yellow asterisk) was lysed using physical manipulation (see Supplementary Experimental Procedures). Prior to manipulation, cells were treated with 200 μM LatA (actin inhibitor) or DMSO (control). Cells were stained with blankophor to visualize the deformed septum. D) Cell-cell separation and new end formation are actin independent. Septated cells were treated with 200 μM LatA or DMSO at time 0 and stained with blankophor at 25 min. Brightfield and fluorescence images at indicated time points are shown. E) New end formation and cell separation are independent of wall synthesis. Septated cells were treated with 20 μg/ml caspofungin at time 0, and imaged in bright-field. Scale bars = 4 μm. In this work, all values with errors are MEAN ± SD.
Figure 2. Determining the mechanical properties of the cell wall
A) Model of the fission yeast cell wall as a thin elastic capsule that is inflated by turgor pressure in the natural state (intact cell), and shrinks to a relaxed state when pressure difference is lost. B) Left panel shows a bright-field image of an individual cell before and after lysis by laser microsurgery (Movie 3). Right panel shows a ghost cell (cell wall only). C) Measurement of expansion ratios. Cells were lysed by multiple approaches. Dimensions of individual cells were measured before and after lysis. Top graph shows expansion ratios of cell lengths (%L∗=L1-L0L0×100) and widths (%R∗=R1-R0R0×100). Bottom graph shows ratios between expansion ratios. For ghosts, the length expansion ratio cannot be measured since the prior geometry of the cells is not known. D) Dimensions of individual cells exposed to various sorbitol concentrations were measured. Graphs show experimental and theoretical osmotic response ratios for length (%L≡L1-L0Lx×100) and width (%R≡R1-RxRx×100) where Lx and Rx are dimensions after the shrinkage. β is the water inaccessible volume fraction. E) Ratio between the response ratios. Scale bars = 4 μm.
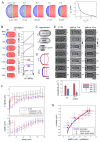
Figure 3. Modeling new end formation
A) Output of simulations in which turgor pressure causes bulging of the new end cell wall. Simulations are shown in which Ys (Young’s modulus of SS) is treated as a free parameter while Δ_P =_ 1.5 MPa and Y = 50 MPa. Graph shows the amount of bulging (s_1) plotted as a function of Ys/Y for measured average SS thickness ts = 100 nm. B) Simulation of a whole cell at varying values of pressure Δ_P where Y = 50 MPa and Ys = Y/2 = 25 MPa. C) Images of a single cell stained with Lectin-TRITC in intact, lysed, and ghost states, which were generated by successive micromanipulations. D) Averaged NE shapes (from C; n=8 cells) were fitted to an ellipse-rectangle combination and compared to simulation outputs (blue dots). Note that the NE in ghosts does not return to a completely flat geometry (Figure S3B) because of the shrinkage of the lateral cell wall, as also seen in the simulations. The slight discrepancies (maximum error < 0.2 μm) between the ghost profiles could be due to additional structural features between the septum and lateral wall not included in the simulations [12]. Scale bar=1 μm. E) Effect of sorbitol treatment on NE shape. Fully septated cells were treated with 1.3 M sorbitol at 1 or 15 min (arrows) after the time of rupture (t=0). Graph shows curvatures of the OE and NE from “shift at 15 m” experiment and simulations (circles; Figure 3B). F) NE bulging (s values) and cell widths as a function of time after sorbitol treatment. As indicated by their width, cells initially shrink and then re-inflate over 60 min to their original size without tip growth (Figure S3C). G) Relationship between NE bulging and cell width (as an indicator of turgor pressure). Data from experiments in Figure 3C,E or F are plotted and compared to simulation outputs from Figure 3B.
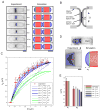
Figure 4. Modeling cell separation and septum bulging
A, B) Images and simulations of cell separation. Experimental images of sister cells stained with blankophor (Movie 5) are compared to outputs from simulations where P = 1.5 MPa and Y = 50 MPa. The PS is assumed to be an elastic disc with Young’s modulus Yp that gradually decreases in diameter, causing the gradual deformation of the SS. Lateral cell wall thickness t ≈ 200 nm, SS thickness ts ≈ 100 nm and the PS thickness tp ≈ 50nm. C) Bulging of the SS (_s_4) as a function of PS diameter (D). Graph compares experimental data (black dots and red points) with simulations for different values of Yp and Ys. The best fits to experimental data are simulations with Ys = Y/2 and 0 ≤ Yp ≤ Y. D) The deformation of the whole septum after laser ablation (as in Figure 1B). Inverse image of a blankofluor-stained cell and the output of a simulation. E) Comparison of experiments and simulations for NE formation (s_1) and whole septum deformations (s_5). The whole septum simulations are done with P = 1.5 MPa and Y = 50 MPa; Ys = Y/2 and Yp = 0,Y,2_Y, and 5_Y.
Similar articles
- Forces that shape fission yeast cells.
Chang F. Chang F. Mol Biol Cell. 2017 Jul 7;28(14):1819-1824. doi: 10.1091/mbc.E16-09-0671. Mol Biol Cell. 2017. PMID: 28684607 Free PMC article. - Mechanics and morphogenesis of fission yeast cells.
Davì V, Minc N. Davì V, et al. Curr Opin Microbiol. 2015 Dec;28:36-45. doi: 10.1016/j.mib.2015.07.010. Epub 2015 Aug 26. Curr Opin Microbiol. 2015. PMID: 26291501 Review. - Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast.
Proctor SA, Minc N, Boudaoud A, Chang F. Proctor SA, et al. Curr Biol. 2012 Sep 11;22(17):1601-8. doi: 10.1016/j.cub.2012.06.042. Epub 2012 Jul 26. Curr Biol. 2012. PMID: 22840513 Free PMC article. - Systematic mapping of cell wall mechanics in the regulation of cell morphogenesis.
Davì V, Chevalier L, Guo H, Tanimoto H, Barrett K, Couturier E, Boudaoud A, Minc N. Davì V, et al. Proc Natl Acad Sci U S A. 2019 Jul 9;116(28):13833-13838. doi: 10.1073/pnas.1820455116. Epub 2019 Jun 24. Proc Natl Acad Sci U S A. 2019. PMID: 31235592 Free PMC article. - Regulation of contractile ring formation and septation in Schizosaccharomyces pombe.
Willet AH, McDonald NA, Gould KL. Willet AH, et al. Curr Opin Microbiol. 2015 Dec;28:46-52. doi: 10.1016/j.mib.2015.08.001. Epub 2015 Sep 3. Curr Opin Microbiol. 2015. PMID: 26340438 Free PMC article. Review.
Cited by
- Control of nuclear size by osmotic forces in Schizosaccharomyces pombe.
Lemière J, Real-Calderon P, Holt LJ, Fai TG, Chang F. Lemière J, et al. Elife. 2022 Jul 20;11:e76075. doi: 10.7554/eLife.76075. Elife. 2022. PMID: 35856499 Free PMC article. - Characterizing non-exponential growth and bimodal cell size distributions in fission yeast: An analytical approach.
Jia C, Singh A, Grima R. Jia C, et al. PLoS Comput Biol. 2022 Jan 18;18(1):e1009793. doi: 10.1371/journal.pcbi.1009793. eCollection 2022 Jan. PLoS Comput Biol. 2022. PMID: 35041656 Free PMC article. - How do fission yeast cells grow and connect growth to the mitotic cycle?
Sveiczer Á, Horváth A. Sveiczer Á, et al. Curr Genet. 2017 May;63(2):165-173. doi: 10.1007/s00294-016-0632-0. Epub 2016 Jul 27. Curr Genet. 2017. PMID: 27465359 Review. - Calcium spikes accompany cleavage furrow ingression and cell separation during fission yeast cytokinesis.
Poddar A, Sidibe O, Ray A, Chen Q. Poddar A, et al. Mol Biol Cell. 2021 Jan 1;32(1):15-27. doi: 10.1091/mbc.E20-09-0609. Epub 2020 Nov 11. Mol Biol Cell. 2021. PMID: 33175606 Free PMC article. - Rapid adaptation of endocytosis, exocytosis, and eisosomes after an acute increase in membrane tension in yeast cells.
Lemière J, Ren Y, Berro J. Lemière J, et al. Elife. 2021 May 13;10:e62084. doi: 10.7554/eLife.62084. Elife. 2021. PMID: 33983119 Free PMC article.
References
- Boudaoud A. Growth of walled cells: from shells to vesicles. Phys Rev Letters. 2003;91:018104. - PubMed
- Campas O, Mahadevan L. Shape and dynamics of tip-growing cells. Curr Biol. 2009;19:2102–2107. - PubMed
- Campas O, Rojas E, Dumais J, Mahadevan L. Strategies for cell shape control in tip-growing cells. Am J Bot. 2012;99:1577–1582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources