Alzheimer's disease cerebrospinal fluid biomarker in cognitively normal subjects - PubMed (original) (raw)
. 2015 Sep;138(Pt 9):2701-15.
doi: 10.1093/brain/awv199. Epub 2015 Jul 27.
Henrik Zetterberg 2, Argonde C van Harten 3, Lidia Glodzik 4, Pablo Martinez-Lage 5, Luisella Bocchio-Chiavetto 6, Lorena Rami 7, Oskar Hansson 8, Reisa Sperling 9, Sebastiaan Engelborghs 10, Ricardo S Osorio 4, Hugo Vanderstichele 11, Manu Vandijck 12, Harald Hampel 13, Stefan Teipl 14, Abhay Moghekar 15, Marilyn Albert 15, William T Hu 16, Jose A Monge Argilés 17, Ana Gorostidi 18, Charlotte E Teunissen 19, Peter P De Deyn 10, Bradley T Hyman 20, Jose L Molinuevo 7, Giovanni B Frisoni 21, Gurutz Linazasoro 5, Mony J de Leon 4, Wiesje M van der Flier 22, Philip Scheltens 3, Kaj Blennow 23, Leslie M Shaw 1, John Q Trojanowski 24; Alzheimer’s Disease Neuroimaging Initiative
Collaborators, Affiliations
- PMID: 26220940
- PMCID: PMC4643624
- DOI: 10.1093/brain/awv199
Alzheimer's disease cerebrospinal fluid biomarker in cognitively normal subjects
Jon B Toledo et al. Brain. 2015 Sep.
Abstract
In a large multicentre sample of cognitively normal subjects, as a function of age, gender and APOE genotype, we studied the frequency of abnormal cerebrospinal fluid levels of Alzheimer's disease biomarkers including: total tau, phosphorylated tau and amyloid-β1-42. Fifteen cohorts from 12 different centres with either enzyme-linked immunosorbent assays or Luminex® measurements were selected for this study. Each centre sent nine new cerebrospinal fluid aliquots that were used to measure total tau, phosphorylated tau and amyloid-β1-42 in the Gothenburg laboratory. Seven centres showed a high correlation with the new Gothenburg measurements; therefore, 10 cohorts from these centres are included in the analyses here (1233 healthy control subjects, 40-84 years old). Amyloid-β amyloid status (negative or positive) and neurodegeneration status (negative or positive) was established based on the pathological cerebrospinal fluid Alzheimer's disease cut-off values for cerebrospinal fluid amyloid-β1-42 and total tau, respectively. While gender did not affect these biomarker values, APOE genotype modified the age-associated changes in cerebrospinal fluid biomarkers such that APOE ε4 carriers showed stronger age-related changes in cerebrospinal fluid phosphorylated tau, total tau and amyloid-β1-42 values and APOE ε2 carriers showed the opposite effect. At 40 years of age, 76% of the subjects were classified as amyloid negative, neurodegeneration negative and their frequency decreased to 32% at 85 years. The amyloid-positive neurodegeneration-negative group remained stable. The amyloid-negative neurodegeneration-positive group frequency increased slowly from 1% at 44 years to 16% at 85 years, but its frequency was not affected by APOE genotype. The amyloid-positive neurodegeneration-positive frequency increased from 1% at 53 years to 28% at 85 years. Abnormally low cerebrospinal fluid amyloid-β1-42 levels were already frequent in midlife and APOE genotype strongly affects the levels of cerebrospinal fluid amyloid-β1-42, phosphorylated tau and total tau across the lifespan without influencing the frequency of subjects with suspected non-amyloid pathology.
Keywords: Alzheimer’s disease; biomarkers; cognitive ageing; dementia; imaging.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
In a large multicentre study, Toledo et al. examine core Alzheimer’s disease CSF biomarkers in 1233 cognitively normal subjects aged 40–85 years. Alzheimer disease-like changes in Aβ1-42 are seen as early as middle age, while APOE genotype strongly modifies age-related effects on both Aβ1–42 and phosphorylated/total tau.
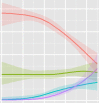
Figure 1
CSF amyloid-β1-42, total tau and phosphorylated tau181 levels in association with ageing in healthy controls stratified by APOE genotype. Dashed lines represent the cut-off points for the biomarkers.
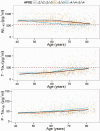
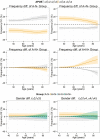
Figure 2
Estimated frequency of pathological amyloid-β (A) and neurodegeneration (N) categories according to age of the subjects. Plots represent all subjects and subjects stratified by gender and APOE genotype. Due to smaller sample size subjects with ε2 alleles were not stratified by gender. Shaded areas represent 95% CI.
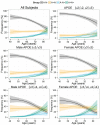
Figure 3
Frequency of A+, N+, A+N− and A+N+ stratified by _APOE_-defined groups.
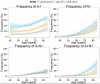
Figure 4
Differences in the frequency of the four biomarker groups in subjects with APOE ε3/ ε3 genotype compared subjects who are ε2 or ε4 allele carriers. The lines above the black dashed line indicate that the plotted group has a higher frequency of the studied biomarker category. For the gender plots values above the 0 represent a higher frequency for females, whereas values below 0 represent a higher frequency in males. Shaded areas represent 95% CI.
Similar articles
- Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia.
Skillbäck T, Farahmand BY, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B, Schott JM, Blennow K, Eriksdotter M, Zetterberg H. Skillbäck T, et al. Brain. 2015 Sep;138(Pt 9):2716-31. doi: 10.1093/brain/awv181. Epub 2015 Jun 30. Brain. 2015. PMID: 26133663 - A data-driven model of biomarker changes in sporadic Alzheimer's disease.
Young AL, Oxtoby NP, Daga P, Cash DM, Fox NC, Ourselin S, Schott JM, Alexander DC; Alzheimer’s Disease Neuroimaging Initiative. Young AL, et al. Brain. 2014 Sep;137(Pt 9):2564-77. doi: 10.1093/brain/awu176. Epub 2014 Jul 9. Brain. 2014. PMID: 25012224 Free PMC article. - Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer's disease.
Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, Trojanowski JQ, Zetterberg H, Blennow K, Weiner MW; Alzheimer's Disease Neuroimaging Initiative*. Mattsson N, et al. Brain. 2015 Mar;138(Pt 3):772-83. doi: 10.1093/brain/awu367. Epub 2014 Dec 24. Brain. 2015. PMID: 25541191 Free PMC article. - Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.
Kang JH, Vanderstichele H, Trojanowski JQ, Shaw LM. Kang JH, et al. Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review. - Cerebrospinal fluid biomarkers of Alzheimer's disease.
Fagan AM, Holtzman DM. Fagan AM, et al. Biomark Med. 2010 Feb;4(1):51-63. doi: 10.2217/BMM.09.83. Biomark Med. 2010. PMID: 20361010 Free PMC article. Review.
Cited by
- Non-linear Relationship Between Plasma Amyloid-β 40 Level and Cognitive Decline in a Cognitively Normal Population.
Gao F, Shang S, Chen C, Dang L, Gao L, Wei S, Wang J, Huo K, Deng M, Wang J, Qu Q. Gao F, et al. Front Aging Neurosci. 2020 Sep 11;12:557005. doi: 10.3389/fnagi.2020.557005. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33061905 Free PMC article. - PKCδ serves as a potential biomarker and therapeutic target for microglia-mediated neuroinflammation in Alzheimer's disease.
Du Y, Guo T, Hao Y, Li C, Tang L, Li X, Zhang X, Li L, Yao D, Xu X, Si H, Zhang J, Zhao N, Yu T, Zhao Y, Zhang W, Xu H. Du Y, et al. Alzheimers Dement. 2024 Aug;20(8):5511-5527. doi: 10.1002/alz.14047. Epub 2024 Jun 28. Alzheimers Dement. 2024. PMID: 38938161 Free PMC article. - Longitudinal CSF Alzheimer's disease biomarker changes from middle age to late adulthood.
Pettigrew C, Soldan A, Wang J, Wang MC, Greenberg B, Albert M, Moghekar A; BIOCARD Research Team. Pettigrew C, et al. Alzheimers Dement (Amst). 2022 Nov 18;14(1):e12374. doi: 10.1002/dad2.12374. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 36415591 Free PMC article. - Don't forget about tau: the effects of ApoE4 genotype on Alzheimer's disease cerebrospinal fluid biomarkers in subjects with mild cognitive impairment-data from the Dementia Competence Network.
Benson GS, Bauer C, Hausner L, Couturier S, Lewczuk P, Peters O, Hüll M, Jahn H, Jessen F, Pantel J, Teipel SJ, Wagner M, Schuchhardt J, Wiltfang J, Kornhuber J, Frölich L. Benson GS, et al. J Neural Transm (Vienna). 2022 Jun;129(5-6):477-486. doi: 10.1007/s00702-022-02461-0. Epub 2022 Jan 21. J Neural Transm (Vienna). 2022. PMID: 35061102 Free PMC article. - Multimodal characterization of older APOE2 carriers reveals selective reduction of amyloid load.
Grothe MJ, Villeneuve S, Dyrba M, Bartrés-Faz D, Wirth M; Alzheimer's Disease Neuroimaging Initiative. Grothe MJ, et al. Neurology. 2017 Feb 7;88(6):569-576. doi: 10.1212/WNL.0000000000003585. Epub 2017 Jan 6. Neurology. 2017. PMID: 28062720 Free PMC article.
References
- Berenguer RG, Monge Argiles JA, Ruiz CM, Paya JS, Blanco Canto MA, Santana CL. Alzheimer disease cerebrospinal fluid biomarkers predict cognitive decline in healthy elderly over 2 years. Alzheimer Dis Assoc Disord 2014; 28: 234–8. - PubMed
- Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol 2011; 121: 171–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG022374/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- CAPMC/ CIHR/Canada
- P50 NS053488/NS/NINDS NIH HHS/United States
- AG1210/AG/NIA NIH HHS/United States
- AG022374/AG/NIA NIH HHS/United States
- AG13616/AG/NIA NIH HHS/United States
- P50 AG016976/AG/NIA NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- K23 AG042856/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- R01 HL118624/HL/NHLBI NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG033655/AG/NIA NIH HHS/United States
- R21 AG043885/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P01 AG032953/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous