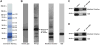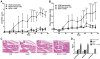Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis - PubMed (original) (raw)
. 2015 Nov;67(11):2877-87.
doi: 10.1002/art.39283.
Christopher Rhodes 1, Kazuhiro Onuma 1, Xiaoyan Zhao 1, Orr Sharpe 1, Tal Gazitt 1, Rani Shiao 1, Justyna Fert-Bober 2, Danye Cheng 1, Lauren J Lahey 1, Heidi H Wong 1, Jennifer Van Eyk 2, William H Robinson 1, Jeremy Sokolove 1
Affiliations
- PMID: 26227989
- PMCID: PMC4626401
- DOI: 10.1002/art.39283
Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis
Dong Hyun Sohn et al. Arthritis Rheumatol. 2015 Nov.
Abstract
Objective: Anti-citrullinated protein antibodies (ACPAs) are characteristic of rheumatoid arthritis (RA). However, their presence years before the onset of clinical RA is perplexing. Although multiple putative citrullinated antigens have been identified, no studies have demonstrated the specific capacity of these antigens to initiate inflammatory arthritis. This study was undertaken to recapitulate the transition from preclinical to clinical RA and to demonstrate the capacity of local citrullination to facilitate this transition.
Methods: We performed proteomic analysis of activated human neutrophils to identify citrullinated proteins, including those targeted as part of the RA immune response. Using enzyme-linked immunosorbent assay, we compared RA and osteoarthritis synovial fluid for levels of citrullinated histone H2B and its immune complex. Using macrophage activation assays, we assessed the effect of histone citrullination on immunostimulatory capacity and evaluated the stimulatory capacity of native and citrullinated H2B immune complexes. Finally, we assessed the potential for anti-citrullinated H2B antibodies to mediate arthritis in vivo.
Results: We identified robust targeting of neutrophil-derived citrullinated histones by the ACPA immune response. More than 90% of the RA patients had anti-citrullinated H2B antibodies. Histone citrullination increased innate immunostimulatory capacity, and immune complexes containing citrullinated histones activated macrophage cytokine production and propagated neutrophil activation. Finally, we demonstrated that immunization with H2B was arthritogenic, but only in the setting of underlying articular inflammation.
Conclusion: Our findings indicate that citrullinated histones, specifically citrullinated H2B, are an antigenic target of the ACPA immune response. Furthermore, local generation of citrullinated antigen during low-grade articular inflammation provides a mechanistic model for the conversion from preclinical autoimmunity to inflammatory arthritis.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Figures
Figure 1
Proteomic analysis of the products of neutrophil activation for targeting by the RA immune response. A and B, neutrophil activation was induced by 10 μM ionomycin, and protein products separated by SDS-PAGE and (A) stained with Coomassie blue, (B) immunoblotted with healthy IgG, isolated RA-IgG, citrullinated residues were acid modified followed by immunoblotting with anti-modified citrulline antibody, or immunoblotted with anti-H2B antibody. Mass spectroscopic identification of proteins in (A) provided in Supplementary Table 1. C and D, Products of neutrophil activation were immunoprecipitated with human RA-IgG (C) or anti-H2B antibody (D) and immunoblotted with anti-modified citrulline antibody or anti-H2B antibody. NB: Band #5 with prominent staining by RA-IgG on (B) was confirmed to be micrococcal nuclease (thermonuclease; S. aureus) used in the digestion of neutrophil activation products.
Figure 2
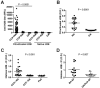
Prominent targeting of citrullinated H2B by RA-associated autoantibodies. A, Bead-based immunoassay analysis of anti-citrullinated and anti-native H2B autoantibodies in anti-CCP2+ RA (n = 81), anti-CCP2- RA (n= 85), and OA (n = 36) patient plasma. B, Citrullinated H2B ELISA analysis of RA (n = 19) and OA (n = 13) synovial fluids. C and D, Analysis of H2B immune complexes in anti-CCP2+ RA (n = 20), anti-CCP2- RA (n = 10), and PsA (n = 14) plasma (C), or RA (n = 14), OA (n = 10), and PsA (n = 4) synovial fluid (D).
Figure 3
Immunization with cH2B induces inflammatory arthritis in the presence of low-grade joint inflammation. A and B, Arthritis score (A) and paw swelling (B) over time in mice immunized with adjuvant alone (CFA), cH2B alone (cH2B), low dose type II collagen (ldCIA), or a combination of low dose collagen and cH2B (ldCIA + cH2B) (n = 5 per group). C, Representative images of H&E-stained sections of ankle joints from mice described in A and B. Scale bar, 500 μm. D, Histological scores of inflammation, pannus formation, and bone or cartilage erosions of ankle joints from mice described above. * = P < 0.05; *** = P < 0.001 compared with CFA control.
Figure 4
Assessment of immunoreactivity developed after immunization with cH2B and/or low dose type II collagen. A, Similar levels of anti-cH2B and anti-CII antibodies on day 17 and at experimental termination (day 50) in mice receiving single or co-immunization. B and C, Multiplex immunoassay was performed to measure a panel of citrullinated antigens and their non-citrullinated controls. Significance analysis of microarray comparison between groups identified ACPA subtypes elevated before boost (day 17) (B) and at experimental termination (day 50) (C) (n = 9 ∼ 10 per group). D, Serum transferred from cH2B immunized mice exacerbates arthritis compared with serum from mice immunized with adjuvant alone (n = 6 per group).
Figure 5
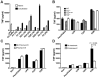
Effect of histone citrullination on innate immunostimulatory capacity. A-D, ELISA quantification of TNFα production from (A) human macrophages stimulated with native or citrullinated histones, (B) murine macrophage deficient in TLR2, TLR4, or TLR9, (C) murine macrophage stimulated with H2B in the presence of polymyxin B or (D) with H2B after treatment with proteinase K and boiling.
Figure 6
Citrullinated histone-containing immune complexes stimulate macrophage activation and propagate neutrophil activation. A, ELISA quantification of human macrophage TNFα production in response to native or citrullinated histone immune complex generated with a polyclonal anti-H2B antibody. B, ELISA quantification of human macrophage TNFα production in response to citrullinated H2B immune complex (cH2B-IC) generated with human RA patient derived IgG (RA-IgG) with blockade of FcγRIIa (FcB), TLR4 (TLR4B), or both. C, ELISA quantitation of serum TNFα levels from RA patients across tertiles of anti-cH2B antibody levels. D, Sytox green quantitation of neutrophil activation in response to cH2B-IC generated with human RA-IgG, or native and citrullinated H2B immune complexes generated with polyclonal anti-H2B antibody.
Comment in
- Rheumatoid arthritis: Autoantibodies, citrullinated histones and initiation of synovitis.
Deane KD. Deane KD. Nat Rev Rheumatol. 2015 Dec;11(12):688-9. doi: 10.1038/nrrheum.2015.134. Epub 2015 Sep 29. Nat Rev Rheumatol. 2015. PMID: 26416595 No abstract available.
Similar articles
- Characterization and Localization of Citrullinated Proteoglycan Aggrecan in Human Articular Cartilage.
Glant TT, Ocsko T, Markovics A, Szekanecz Z, Katz RS, Rauch TA, Mikecz K. Glant TT, et al. PLoS One. 2016 Mar 4;11(3):e0150784. doi: 10.1371/journal.pone.0150784. eCollection 2016. PLoS One. 2016. PMID: 26943656 Free PMC article. - Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs.
Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, Pitzalis C. Corsiero E, et al. Ann Rheum Dis. 2016 Oct;75(10):1866-75. doi: 10.1136/annrheumdis-2015-208356. Epub 2015 Dec 9. Ann Rheum Dis. 2016. PMID: 26659717 Free PMC article. - Citrullinated Autoantigens: From Diagnostic Markers to Pathogenetic Mechanisms.
Muller S, Radic M. Muller S, et al. Clin Rev Allergy Immunol. 2015 Oct;49(2):232-9. doi: 10.1007/s12016-014-8459-2. Clin Rev Allergy Immunol. 2015. PMID: 25355199 Review. - Citrullination of synovial proteins in murine models of rheumatoid arthritis.
Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, van den Berg WB, van Venrooij WJ, Joosten LA. Vossenaar ER, et al. Arthritis Rheum. 2003 Sep;48(9):2489-500. doi: 10.1002/art.11229. Arthritis Rheum. 2003. PMID: 13130468 - Insights into the study and origin of the citrullinome in rheumatoid arthritis.
Fert-Bober J, Darrah E, Andrade F. Fert-Bober J, et al. Immunol Rev. 2020 Mar;294(1):133-147. doi: 10.1111/imr.12834. Epub 2019 Dec 25. Immunol Rev. 2020. PMID: 31876028 Free PMC article. Review.
Cited by
- CHHM: a Manually Curated Catalogue of Human Histone Modifications Revealing Hotspot Regions and Unique Distribution Patterns.
Ma W, Ding X, Xu J, Poon TCW. Ma W, et al. Int J Biol Sci. 2024 Jul 2;20(10):3760-3772. doi: 10.7150/ijbs.95954. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113691 Free PMC article. - The interplay between citrullination and HLA-DRB1 polymorphism in shaping peptide binding hierarchies in rheumatoid arthritis.
Ting YT, Petersen J, Ramarathinam SH, Scally SW, Loh KL, Thomas R, Suri A, Baker DG, Purcell AW, Reid HH, Rossjohn J. Ting YT, et al. J Biol Chem. 2018 Mar 2;293(9):3236-3251. doi: 10.1074/jbc.RA117.001013. Epub 2018 Jan 9. J Biol Chem. 2018. PMID: 29317506 Free PMC article. - B cells in rheumatoid arthritis synovial tissues encode focused antibody repertoires that include antibodies that stimulate macrophage TNF-α production.
Elliott SE, Kongpachith S, Lingampalli N, Adamska JZ, Cannon BJ, Blum LK, Bloom MS, Henkel M, McGeachy MJ, Moreland LW, Robinson WH. Elliott SE, et al. Clin Immunol. 2020 Mar;212:108360. doi: 10.1016/j.clim.2020.108360. Epub 2020 Feb 5. Clin Immunol. 2020. PMID: 32035179 Free PMC article. - Associations of Smoking and Age With Inflammatory Joint Signs Among Unaffected First-Degree Relatives of Rheumatoid Arthritis Patients: Results From Studies of the Etiology of Rheumatoid Arthritis.
Sparks JA, Chang SC, Deane KD, Gan RW, Kristen Demoruelle M, Feser ML, Moss L, Buckner JH, Keating RM, Costenbader KH, Gregersen PK, Weisman MH, Mikuls TR, O'Dell JR, Michael Holers V, Norris JM, Karlson EW. Sparks JA, et al. Arthritis Rheumatol. 2016 Aug;68(8):1828-38. doi: 10.1002/art.39630. Arthritis Rheumatol. 2016. PMID: 26866831 Free PMC article. - Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis.
Catrina AI, Svensson CI, Malmström V, Schett G, Klareskog L. Catrina AI, et al. Nat Rev Rheumatol. 2017 Feb;13(2):79-86. doi: 10.1038/nrrheum.2016.200. Epub 2016 Dec 15. Nat Rev Rheumatol. 2017. PMID: 27974851 Review.
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. - PubMed
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. - PubMed
- Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. - PubMed
- Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR063676/AR/NIAMS NIH HHS/United States
- IK2 BX001301/BX/BLRD VA/United States
- N01 HV028183/HV/NHLBI NIH HHS/United States
- R01 AI085268/AI/NIAID NIH HHS/United States
- R01-AR-063676/AR/NIAMS NIH HHS/United States
- N01-HV-28183/HV/NHLBI NIH HHS/United States
- R01-AI-085268/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical