Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells - PubMed (original) (raw)
Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells
Lucas B Sullivan et al. Cell. 2015.
Abstract
Mitochondrial respiration is important for cell proliferation; however, the specific metabolic requirements fulfilled by respiration to support proliferation have not been defined. Here, we show that a major role of respiration in proliferating cells is to provide electron acceptors for aspartate synthesis. This finding is consistent with the observation that cells lacking a functional respiratory chain are auxotrophic for pyruvate, which serves as an exogenous electron acceptor. Further, the pyruvate requirement can be fulfilled with an alternative electron acceptor, alpha-ketobutyrate, which provides cells neither carbon nor ATP. Alpha-ketobutyrate restores proliferation when respiration is inhibited, suggesting that an alternative electron acceptor can substitute for respiration to support proliferation. We find that electron acceptors are limiting for producing aspartate, and supplying aspartate enables proliferation of respiration deficient cells in the absence of exogenous electron acceptors. Together, these data argue a major function of respiration in proliferating cells is to support aspartate synthesis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
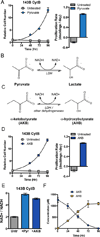
Figure 1. Cytochrome B Mutant 143B Cybrid Cells are Auxotrophic for Electron Acceptors that can Regenerate NAD+
(A) Proliferation rate of 143B CytB cells was determined in the presence or absence of pyruvate. (left) Cell counts, normalized to cell number at t=0 when media conditions were applied, were assessed over time and used to calculate proliferation rate (right). (B) Pyruvate is a substrate of lactate dehydrogenase (LDH), accepting electrons from NADH to produce NAD+ and lactate. (C) Alpha-ketobutyrate (AKB) can also act as an electron acceptor from NADH and yield NAD+ and alpha-hydroxybutyrate (AHB). (D) Proliferation rate for 143B CytB cells in the presence or absence of AKB was determined as in (A). (E) Intracellular ratio of NAD+/NADH was determined in 143B CytB cells in untreated media or in the presence of pyruvate (Pyr) or AKB. (F) The concentration of AKB and AHB in the media of 143B CytB cells cultured in the presence of AKB was determined over time by GCMS analysis. Values in all figure panels denote mean ± standard error of the mean (SEM), n=3. See also Figure S1.
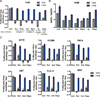
Figure 2. Proliferation of Respiration-Inhibited Cells is Restored by Exogenous Electron Acceptors
(A) The proliferation rate of 143B cells was determined in media with or without AKB supplementation in the absence (Untr) or in the presence of the mitochondrial respiration inhibitors rotenone (Rot), phenformin (Phen), antimycin (Ant), myxothiazol (Myxo), azide (N3), or oligomycin (Oligo). (B) The ratio of NAD+/NADH was determined in 143B cells with or without AKB supplementation in the absence or presence of respiration inhibitors as in (A). (C) The proliferation rate of A172, H1299, HeLa, U87, FL5.12, and MEF cell lines was determined with or without AKB supplementation in the absence or presence of rotenone, antimycin, or oligomycin. Values in all figure panels denote mean ± SEM, n=3. See also Figure S2.
Figure 3. Oxygen Utilization in the Absence of Mitochondrial ATP Production is Sufficient for Cell Proliferation
(A) Schematic illustrating the effects of oligomycin and FCCP on mitochondrial membrane potential and ATP synthesis. Oligomycin treatment inhibits ATP synthase, resulting in a hyperpolarized mitochondrial membrane. This hyperpolarization inhibits proton pumping and thereby inhibits ETC activity resulting in decreased NADH oxidation and O2 consumption (left). Treatment with FCCP in addition to oligomycin relieves the hyperpolarization of the mitochondrial membrane allowing restoration of NADH oxidation and mitochondrial O2 consumption without restoring ATP production (right). (B) Proliferation rate of 143B cells treated with oligomycin in the presence or absence of FCCP treatment. (C) Mitochondrial oxygen consumption rate of 143B cells treated with oligomycin with or without FCCP. (D) Intracellular NAD+/NADH ratio in 143B cells treated with oligomycin in the presence or absence of FCCP. Values in all figure panels denote mean ± SEM, n=3 (B, D), n=5 (C).
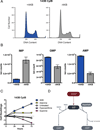
Figure 4. Electron Acceptor Insufficiency Affects Purine Nucleotide Levels
(A) Analysis of DNA content by propidium iodide staining and flow cytometry of 143B CytB cells cultured with our without AKB supplementation. (B) LCMS quantification of purine nucleotide levels in 143B CytB cells cultured with or without AKB supplementation. (C) Cell doublings were measured over time of 143B CytB cells cultured in unsupplemented media or media supplemented with AKB, adenine, hypoxanthine, or guanine. (D) Schematic illustrating the use of IMP for GMP and AMP synthesis. Synthesis of GMP uses NAD+, whereas synthesis of AMP requires aspartate. Values in (B) and (C) denote mean ± SEM, n=3. See also Figure S3.
Figure 5. Electron Acceptor Insufficiency Suppresses TCA Metabolite and Aspartate Levels
(A) Schematic detailing the TCA cycle reaction routes for the biosynthesis of aspartate from glutamine. (B) Isotopomer distribution of aspartate from 143B CytB cells supplemented with AKB and 143B cells cultured in the presence of U-13C Glutamine for 8 hours. (C) GCMS quantification of TCA cycle metabolites, glutamate, and aspartate from 143B CytB cells cultured with or without AKB. (D) GCMS quantification of TCA metabolites, glutamate, and aspartate from 143B cells cultured with or without AKB in the presence or absence of the indicated mitochondrial inhibitors. Ion counts are relative to untreated 143B cells, which is denoted by the dashed grey line in each panel. Values denote mean ± SEM, n=3. See also Figure S4.
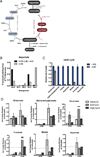
Figure 6. Aspartate is the Key Biosynthetic Precursor Provided by Respiration
(A) Proliferation rate of 143B CytB cells determined in the presence or absence of aspartate. (B) The proliferation rate of 143B cells cultured with or without aspartate in the presence of the mitochondrial respiration inhibitors rotenone (Rot), phenformin (Phen), antimycin (Ant), myxothiazol (Myxo), azide (N3), or oligomycin (Oligo). (C) The proliferation rate of A172, H1299, HeLa, U87, FL5.12, and MEF cells cultured with or without aspartate in the presence of rotenone, antimycin, or oligomycin. (D) Schematic detailing the role of respiration and exogenous electron acceptors in aspartate biosynthesis. The conversion of nutrients into aspartate requires the removal of electrons and therefore requires access to electron acceptors, which can be supplied by respiration (O2) or exogenous electron acceptors such as AKB. Maintenance of aspartate pools supports nucleotide and protein biosynthesis. Values in all figure panels denote mean ± SEM, n=3. See also Figure S5.
Similar articles
- An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. Birsoy K, et al. Cell. 2015 Jul 30;162(3):540-51. doi: 10.1016/j.cell.2015.07.016. Cell. 2015. PMID: 26232224 Free PMC article. - Aerobic glycolysis: meeting the metabolic requirements of cell proliferation.
Lunt SY, Vander Heiden MG. Lunt SY, et al. Annu Rev Cell Dev Biol. 2011;27:441-64. doi: 10.1146/annurev-cellbio-092910-154237. Annu Rev Cell Dev Biol. 2011. PMID: 21985671 Review. - Synthesis of glutamate and aspartate in rat brain synaptosomes.
Bielarczyk H, Lysiak W, Szutowicz A. Bielarczyk H, et al. Acta Biochim Pol. 1986;33(4):239-51. Acta Biochim Pol. 1986. PMID: 2881415 - Severe impairment of nucleotide synthesis through inhibition of mitochondrial respiration.
Gattermann N, Dadak M, Hofhaus G, Wulfert M, Berneburg M, Loeffler ML, Simmonds HA. Gattermann N, et al. Nucleosides Nucleotides Nucleic Acids. 2004 Oct;23(8-9):1275-9. doi: 10.1081/NCN-200027545. Nucleosides Nucleotides Nucleic Acids. 2004. PMID: 15571245 - An Asp to Strike Out Cancer? Therapeutic Possibilities Arising from Aspartate's Emerging Roles in Cell Proliferation and Survival.
Helenius IT, Madala HR, Yeh JJ. Helenius IT, et al. Biomolecules. 2021 Nov 10;11(11):1666. doi: 10.3390/biom11111666. Biomolecules. 2021. PMID: 34827664 Free PMC article. Review.
Cited by
- The mitochondrial translation machinery as a therapeutic target in Myc-driven lymphomas.
D'Andrea A, Gritti I, Nicoli P, Giorgio M, Doni M, Conti A, Bianchi V, Casoli L, Sabò A, Mironov A, Beznoussenko GV, Amati B. D'Andrea A, et al. Oncotarget. 2016 Nov 8;7(45):72415-72430. doi: 10.18632/oncotarget.11719. Oncotarget. 2016. PMID: 27635472 Free PMC article. - The malate-aspartate shuttle (Borst cycle): How it started and developed into a major metabolic pathway.
Borst P. Borst P. IUBMB Life. 2020 Nov;72(11):2241-2259. doi: 10.1002/iub.2367. Epub 2020 Sep 11. IUBMB Life. 2020. PMID: 32916028 Free PMC article. Review. - In vivo isotope tracing reveals a requirement for the electron transport chain in glucose and glutamine metabolism by tumors.
Pachnis P, Wu Z, Faubert B, Tasdogan A, Gu W, Shelton S, Solmonson A, Rao AD, Kaushik AK, Rogers TJ, Ubellacker JM, LaVigne CA, Yang C, Ko B, Ramesh V, Sudderth J, Zacharias LG, Martin-Sandoval MS, Do D, Mathews TP, Zhao Z, Mishra P, Morrison SJ, DeBerardinis RJ. Pachnis P, et al. Sci Adv. 2022 Sep 2;8(35):eabn9550. doi: 10.1126/sciadv.abn9550. Epub 2022 Aug 31. Sci Adv. 2022. PMID: 36044570 Free PMC article. - Relationship between metabolic reprogramming and drug resistance in breast cancer.
Lv L, Yang S, Zhu Y, Zhai X, Li S, Tao X, Dong D. Lv L, et al. Front Oncol. 2022 Aug 18;12:942064. doi: 10.3389/fonc.2022.942064. eCollection 2022. Front Oncol. 2022. PMID: 36059650 Free PMC article. Review. - On the Relevance of Soft Tissue Sarcomas Metabolic Landscape Mapping.
Esperança-Martins M, F Duarte I, Rodrigues M, Soares do Brito J, López-Presa D, Costa L, Fernandes I, Dias S. Esperança-Martins M, et al. Int J Mol Sci. 2022 Sep 28;23(19):11430. doi: 10.3390/ijms231911430. Int J Mol Sci. 2022. PMID: 36232732 Free PMC article. Review.
References
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345–19350. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007287/GM/NIGMS NIH HHS/United States
- R01 CA168653/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- GG006413/PHS HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- P30CA1405141/CA/NCI NIH HHS/United States
- Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources