Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap - PubMed (original) (raw)
Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap
Aleksander D Szymaniak et al. Dev Cell. 2015.
Abstract
Epithelial cells undergo dynamic polarity changes as organs pattern, but the relationship between epithelial polarity and cell fate is poorly understood. Using the developing lung as a model, we found that distinct alterations in apical-basal polarity dictate airway epithelial differentiation. We demonstrate that Crb3, a Crumbs isoform that determines epithelial apical domain identity, is required for airway differentiation by controlling the localization of the transcriptional regulator Yap. We show that Crb3 promotes the interaction between Yap and the Hippo pathway kinases Lats1/2 at apical cell junctions to induce Yap phosphorylation and cytoplasmic retention, which drive cell differentiation. Loss of Crb3 in developing mouse airways or isolated adult airway progenitors results in unrestricted nuclear Yap activity and consequent cell differentiation defects. Our findings demonstrate that polarity-dependent cues control airway cell differentiation, offering important molecular insights into organ patterning.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
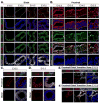
Figure 1. Apical-localized p-YapS112 correlates with polarized Crb3 and Scrib localization as the lung epithelium patterns the proximal domain
(A, B) Immunofluorescence analysis of E12.5–E15.5 mouse lungs examining the levels and localization of Yap (green) and p-YapS112 (red) in the (A) distal and (B) proximal epithelium. The presence of Sox9 (white) served as a marker of distal epithelial cells. (C) Increased Crb3 levels localized to the apical domain are observed specifically in the Sox9-negative proximal region of E12.5 lung epithelium. (D) Scrib levels increase and localize to the basal-lateral surface of E12.5 Sox2-positive proximal lung epithelium. (E, F) High resolution imaging of the transition zone between the Sox9-positive distal to Sox2-positive proximal compartments in the developing lung epithelium reveals an increase in the levels of (E) apical-localized Crb3, and (F) basal-lateral-localized Scrib immediately following the boundary between the Sox2 and Sox9 domains. Of note, this transition zone is precisely where Yap shifts localization from the nucleus to the cytoplasm (Mahoney et al., 2014). DAPI was used to mark the nuclei (blue) in all images, and for clarity the basal surface of the epithelium is outlined with a white dotted line. Scale bar = 10μm.
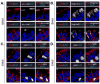
Figure 2. Apical Crb3 and basal-lateral Scrib mark differentiating cells in proximal lung epithelium
Crb3 and Scrib levels were examined by immunofluorescence microscopy in E18.5 mouse lung epithelium, and their pattern of localization was determined in (A) ciliated cells marked by apical acetylated α-tubulin, and (B) secretory cells marked by Scgb1a1 or Scgb1a2 expression. Crb3 and Scrib levels and localization were correlated with p63 expression in (C) E16.5 and (D) E18.5 proximal lung epithelium. Note that p63-positive cells lack Crb3, and display a cortical localization of Scrib. DAPI was used to mark the nuclei (blue) in all images, and the basal surface of the epithelium is outlined with a white dotted line. Scale bar = 2μm.
Figure 3. Apical-Basal Polarity cues correlate with the differentiation of adult airway progenitors
(A) Diagram depicting the differentiation of epithelial progenitors isolated from adult mouse tracheas following cultured in an air-liquid interface (ALI). (B–F) Immunofluorescence confocal miscopy was performed to examine the levels and localization of (B) Yap, (C) F-actin, (D) Crb3, and (E) Scrib in mouse airway basal progenitor cells induced to differentiate in ALI cultures. X-Y views (bottom panels) and the Z-plane apical-basal views (top panels) for each are shown. Basal progenitors, marked by the presence of p63, were examined by immunofluorescence confocal microscopy for the levels and localization of (F) Crb3 and (G) Scrib, and the Z-plane apical-basal view for each is shown. DAPI was used to mark the nuclei (blue) in all images, and in all Z-plane images the nuclei are also outlined with a thin white dotted line. Scale bar = 10μm.
Figure 4. Depletion of Crb3 prevents the differentiation of adult airway epithelial progenitors
Lentiviruses were used to transduce control shRNA (shCTL) or shRNA targeting Crb3 in airway progenitor cells isolated from mouse tracheas, and these cells were subsequently cultured in ALI conditions to induce differentiation. (A) Crb3 and Yap, as well as (B) p63 levels and localization were examined after 6 days of ALI culture by immunofluorescence confocal microscopy. The X-Y view (bottom panels) and the Z-plane apical-basal view (top panels) in (A) are shown, whereas only the Z-plane in (B) is shown. DAPI marked nuclei are outlined with a white dotted line in the Z-plane images. Scale bar = 10μm. (C) Samples obtained from shCTL-or shCrb3-expressing WT or Yap-null airway progenitors cultured for 6 days in ALI cultures were examined by qPCR for expression levels of Crb3, basal progenitor cell markers Tp63 and Krt5, as well as the differentiation markers FoxJ1 (ciliated cells), Scgb1a1 (secretory cells), and Muc5ac (goblet cells) (n=3, average +SEM, ** p<0.0001).
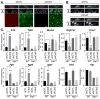
Figure 5. Deletion of Crb3 induces nuclear Yap localization in proximal airways
(A) E18.5 proximal airway sections from wild type (WT) and Crb3-null mice were examined by H&E staining. (B) Proximal airways from the tracheas of WT and Crb3-null mice were analyzed by immunofluorescence microscopy for Crb3 and Yap. (C) The relative levels of nuclear Yap in cells positioned at the basal or luminal side of the epithelium in E18.5 WT and Crb3-null proximal airways was quantitated by determining the Yap pixel intensity/area of DAPI-stained nuclei using Image J software (n=50 from three separate experiments, average +SEM, ** p<0.0001). (D) Intrapulmonary proximal airways were analyzed by immunofluorescence microscopy for Yap and Sox2. (E) Proximal airways from E15.5 and E18.5 WT and Crb3-null lungs were examined for the presence of the proliferation marker Ki67, and (F) the percentage of these respective cells was quantitated (n= 160 to 300 cells from each group, average from three experiments +SEM, ** p<0.0001). The basal epithelial surface is outlined with a white dotted line in all images, and DAPI marked nuclei in the first panels are outlined with a thin white dotted line. Scale bar = 10μm.
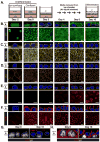
Figure 6. Deletion of Crb3 leads to an aberrant accumulation of undifferentiated basal progenitors in the developing airway
E18.5 proximal airway sections from the tracheas of wild type (WT) and Crb3-null tissues were examined by immunofluorescence microscopy for (A) Krt5 and Yap, (B) Krt5 and p63, (C) Krt5 and Krt8, (D) Krt5 and FoxJ1, (E) Scgb3a2, and (F) Krt5 and Scgb1a1. The basal surface of the epithelium is outlined with a white dotted line, and DAPI marked nuclei in the first panels in experiment (A) are outlined with a thin white dotted line. Scale bar = 10μm. (G) The number of cells expressing the indicated cell fate markers was quantitated and is shown as a percentage of the total population (n=488 from WT, and n=456 from Crb3-null; total from three separate experiments).
Figure 7. Activated Lats1/2 kinases associate with Yap at the apical membrane in differentiated airway epithelial cells
(A) E18.5 proximal airway sections from wild type and Crb3-null mice were examined by immunofluorescence microscopy for total Yap and p-YapS112 (p-Yap), and (B) for p-Lats1/2T1079/T1041 (p-Lats). The basal surface of the epithelium is outlined with a thick white dotted line, and DAPI marked nuclei in the first panels are outlined with a thin white dotted line. Scale bar = 10μm. (C) Cell lysates isolated from undifferentiated Day 0 airway progenitor cells, and ALI-cultured Day 4 and Day 10 differentiated cells were examined by immunoblotting using the indicated antibodies. (D, E) Immunofluorescence confocal microscopy was used to examine the levels and localization of activated (D) p-Lats and (E) p-Yap at different stages of differentiation in ALI-cultured airway progenitors. X-Y images (bottom panels) and the Z-plane apical-basal view (top panels) for each are shown. (F) Disorganized pLats, and (G) a reduction in p-Yap levels, was observed in p63-positive basal airway progenitors cultured under ALI conditions for 0 or 10 days. Z-plane apical-basal view for each is shown. (H) In situ Proximity ligation assay (PLA) followed by confocal microscopy was performed using the indicated antibodies on airway progenitor cells cultured under ALI conditions for the indicated days. Red dots indicate endogenous interactions. (I) In situ PLA followed by confocal microscopy was performed using the indicated antibodies on airway progenitor cells transduced to express shCTL or shCrb3 and grown for 6 days in ALI cultures. For the experiments in (H) and (I) a compressed image of the X-Y stacks is shown to highlight all the PLA spots, as is the Z-plane apical-basal view to highlight the polarization of these spots. DAPI was used to mark the nuclei (blue), and in all Z-plane images the nuclei are also outlined with a thin white dotted line. Scale bar = 10μm for all images. (J) Model for how cell polarity integrates with Hippo pathway signaling to control cell fate in the proximal airways.
Similar articles
- Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung.
Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Lange AW, et al. J Mol Cell Biol. 2015 Feb;7(1):35-47. doi: 10.1093/jmcb/mju046. Epub 2014 Dec 5. J Mol Cell Biol. 2015. PMID: 25480985 Free PMC article. - Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing.
Nantie LB, Young RE, Paltzer WG, Zhang Y, Johnson RL, Verheyden JM, Sun X. Nantie LB, et al. Development. 2018 Nov 9;145(21):dev163105. doi: 10.1242/dev.163105. Development. 2018. PMID: 30305289 Free PMC article. - MUC1-C Represses the Crumbs Complex Polarity Factor CRB3 and Downregulates the Hippo Pathway.
Alam M, Bouillez A, Tagde A, Ahmad R, Rajabi H, Maeda T, Hiraki M, Suzuki Y, Kufe D. Alam M, et al. Mol Cancer Res. 2016 Dec;14(12):1266-1276. doi: 10.1158/1541-7786.MCR-16-0233. Epub 2016 Sep 22. Mol Cancer Res. 2016. PMID: 27658423 Free PMC article. - The Hippo pathway and apico-basal cell polarity.
Genevet A, Tapon N. Genevet A, et al. Biochem J. 2011 Jun 1;436(2):213-24. doi: 10.1042/BJ20110217. Biochem J. 2011. PMID: 21568941 Review. - Epithelial cell polarity, stem cells and cancer.
Martin-Belmonte F, Perez-Moreno M. Martin-Belmonte F, et al. Nat Rev Cancer. 2011 Dec 15;12(1):23-38. doi: 10.1038/nrc3169. Nat Rev Cancer. 2011. PMID: 22169974 Review.
Cited by
- Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity.
Xu P, Londregan A, Rich C, Trinkaus-Randall V. Xu P, et al. Bioengineering (Basel). 2020 Feb 7;7(1):14. doi: 10.3390/bioengineering7010014. Bioengineering (Basel). 2020. PMID: 32046198 Free PMC article. - Cadherin-26 (CDH26) regulates airway epithelial cell cytoskeletal structure and polarity.
Lachowicz-Scroggins ME, Gordon ED, Wesolowska-Andersen A, Jackson ND, MacLeod HJ, Sharp LZ, Sun M, Seibold MA, Fahy JV. Lachowicz-Scroggins ME, et al. Cell Discov. 2018 Feb 13;4:7. doi: 10.1038/s41421-017-0006-x. eCollection 2018. Cell Discov. 2018. PMID: 29449961 Free PMC article. - Pals1 Haploinsufficiency Results in Proteinuria and Cyst Formation.
Weide T, Vollenbröker B, Schulze U, Djuric I, Edeling M, Bonse J, Hochapfel F, Panichkina O, Wennmann DO, George B, Kim S, Daniel C, Seggewiß J, Amann K, Kriz W, Krahn MP, Pavenstädt H. Weide T, et al. J Am Soc Nephrol. 2017 Jul;28(7):2093-2107. doi: 10.1681/ASN.2016040474. Epub 2017 Feb 2. J Am Soc Nephrol. 2017. PMID: 28154200 Free PMC article. - Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine.
Dey A, Varelas X, Guan KL. Dey A, et al. Nat Rev Drug Discov. 2020 Jul;19(7):480-494. doi: 10.1038/s41573-020-0070-z. Epub 2020 Jun 17. Nat Rev Drug Discov. 2020. PMID: 32555376 Free PMC article. Review. - New Insights into Hippo/YAP Signaling in Fibrotic Diseases.
Mia MM, Singh MK. Mia MM, et al. Cells. 2022 Jun 29;11(13):2065. doi: 10.3390/cells11132065. Cells. 2022. PMID: 35805148 Free PMC article. Review.
References
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. - PubMed
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. - PubMed
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL105971/HL/NHLBI NIH HHS/United States
- R01 HL124392-01/HL/NHLBI NIH HHS/United States
- R01 HL105971-01/HL/NHLBI NIH HHS/United States
- R01 HL124392/HL/NHLBI NIH HHS/United States
- R01 HL119836/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases