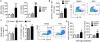Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery After Focal Cerebral Ischemia - PubMed (original) (raw)
Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery After Focal Cerebral Ischemia
Lijuan Han et al. Stroke. 2015 Sep.
Abstract
Background and purpose: Oligodendrogenesis is essential for white matter repair after stroke. Although agonists of peroxisome proliferator-activated receptors γ confer neuroprotection in models of cerebral ischemia, it is not known whether this effect extends to white matter protection. This study tested the hypothesis that the peroxisome proliferator-activated receptors γ agonist rosiglitazone enhances oligodendrogenesis and improves long-term white matter integrity after ischemia/reperfusion.
Methods: Male adult C57/BL6 mice (25-30 g) were subjected to 60-minute middle cerebral artery occlusion and reperfusion. Rosiglitazone (3 mg/kg) was injected intraperitoneally once daily for 14 days beginning 2 hours after reperfusion. Sensorimotor and cognitive functions were evaluated ≤21 days after middle cerebral artery occlusion. Immunostaining was used to assess infarct volume, myelin loss, and microglial activation. Bromodeoxyuridine (BrdU) was injected for measurements of proliferating NG2(+) oligodendrocyte precursor cells (OPCs) and newly generated adenomatous polyposis coli(+) oligodendrocytes. Mixed glial cultures were used to confirm the effect of rosiglitazone on oligodendrocyte differentiation and microglial polarization.
Results: Rosiglitazone significantly reduced brain tissue loss, ameliorated white matter injury, and improved sensorimotor and cognitive functions for at least 21 days after middle cerebral artery occlusion. Rosiglitazone enhanced OPC proliferation and increased the numbers of newly generated mature oligodendrocytes after middle cerebral artery occlusion. Rosiglitazone treatment also reduced the numbers of Iba1(+)/CD16(+) M1 microglia and increased the numbers of Iba1(+)/CD206(+) M2 microglia after stroke. Glial culture experiments confirmed that rosiglitazone promoted oligodendrocyte differentiation, perhaps by promoting microglial M2 polarization.
Conclusions: Rosiglitazone treatment improves long-term white matter integrity after cerebral ischemia, at least, in part, by promoting oligodendrogenesis and facilitating microglial polarization toward the beneficial M2 phenotype.
Keywords: bromodeoxyuridine; inflammation; polarization; stroke; white matter.
© 2015 American Heart Association, Inc.
Figures
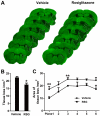
Figure 1. Rosiglitazone Reduces Brain Tissue Loss 21 days after MCAO
A, Representative MAP2-stained coronal sections in vehicle or rosiglitazone-treated mice sacrificed 21 days following MCAO. Dotted lines indicate infarct areas. B, Total volume of brain tissue loss (mm3) in vehicle-treated and rosiglitazone (RSG)-treated mice. C, Infarct areas in 6 consecutive coronal sections throughout the MCA territory, spaced 1 mm apart. N=5-6 per group. Data are expressed as mean ± SEM. * P ≤ 0.05, ** P ≤ 0.01 vs vehicle.
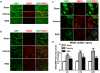
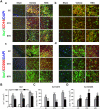
Figure 2. Rosiglitazone Promotes White Matter Integrity 21 days after MCAO
A-C, Representative images of MBP (green) and SMI32 (red) immunostaining in the external capsule (EC, A), cortex (CTX, B) and striatum (STR, C). Scale bar = 50 μm. D, The relative ratio of SMI32 vs MBP immunostaining intensity in the ipsilateral hemisphere was expressed as a function of contralateral values, and expressed as mean ± SEM. N=5-7 per group. *P ≤ 0.05, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ## P ≤ 0.01 vs vehicle.
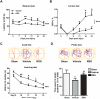
Figure 3. Rosiglitazone Improves Long-term Recovery of Neurological Function after MCAO
A-B, Sensorimotor dysfunction was significantly attenuated in rosiglitazone (RSG)-treated mice up to 21 days after ischemia, as assessed by the Rotarod test (A) and corner test (B). Corner test performance was expressed by the percentage of right turns out of ten turn trials. The performance in the Rotarod test was expressed as the time spent on the rotating rod before falling off. C-D, The Morris water maze test was performed to measure cognitive deficits after cerebral ischemia. C, Latency to find the hidden platform in the cued test (spatial learning). D, Time spent in target quadrant in the probe test (memory consolidation). Data are expressed as mean ± SEM. A-C, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 vs vehicle. D, * P ≤ 0.05 vs sham, # P ≤ 0.05 vs vehicle. N=6-8 per group.
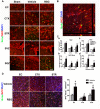
Figure 4. Rosiglitazone Enhances Oligodendrogenesis and Oligodendrocyte Replacement after MCAO
A, Representative images of BrdU (green) and NG2 (red) immunostaining 21 days after cerebral ischemia. Scale bar = 50 μm. B, Representative image showing the colocalization of BrdU, NG2, and DAPI staining at high magnification. Scale bar = 20 μm. C, Numbers of NG2-positive OPCs (a) and BrdU/NG2-dual labeled proliferating OPCs (b) in ipsilateral external capsule (EC), cortex (CTX), and striatum (STR). Numbers of NG2-positive OPCs (c) and BrdU/NG2-dual labeled proliferating OPCs (d) in ipsilateral SVZ and SGZ. D, Rosiglitazone enhances oligodendrocyte replacement after MCAO. Left, Representative images of BrdU (green) and APC (red) immunostaining 21 days after cerebral ischemia in ipsilateral EC, CTX, and STR. Scale bar = 50 μm. Right, numbers of BrdU/APC-dual labeled oligodendrocytes in peri-infarct areas were expressed as cells / mm2. Data are expressed as mean ± SEM. N=6-8 per group. ** P ≤ 0.01, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ### P ≤ 0.001 vs vehicle.
Figure 5. Rosiglitazone Drives M2 Microglial Polarization after MCAO
A, Representative images of Iba1 (green), CD16 (red, a,b), and CD206 (red, c,d) immunostaining 21 days after cerebral ischemia in ipsilateral external capsule (EC), cortex (CTX), and striatum (STR). Scale bar in a, c = 100 μm. Scale bar in b, d = 30 μm. B-D, Numbers of Iba1-positive microglia (B), Iba1/CD16-dual labeled M1 microglia (C) and Iba1/CD206-dual labeled M2 microglia (D) were quantified and expressed as cells / mm2. Data are expressed as mean ± SEM. N=8-10 per group. * P ≤ 0.05, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ## P ≤ 0.01, ### P ≤ 0.001 vs vehicle.
Figure 6. Rosiglitazone Promotes Oligodendrocyte Differentiation and M2 Microglial Polarization in Mixed Glial Cultures
A-C, Mixed glial cultures were treated with 0.5 μM rosiglitazone or DMSO vehicle for 7 days. A, mRNA expression levels of MBP and PLP were measured by real time PCR. B, Flow cytometry analyses of NG2−O4+ differentiating oligodendrocytes in mixed glial cultures. C, mRNA expression of CD206 was measured by real time PCR (Left). Flow cytometry analyses of CD206+CD11b+ microglia in mixed glial cultures. D. Mixed glial cultures were treated with 1.5 mM LME to deplete the microglia and then treated with 0.5 μM rosiglitazone or DMSO vehicle for 7 days. mRNA expression levels of MBP and PLP were measured by real time PCR. Data are expressed as mean ± SEM. N=4/group. *P ≤ 0.05, *** P ≤ 0.001 vs control, # P ≤ 0.05, ## P ≤ 0.01, ### P ≤ 0.001 vs DMSO.
Similar articles
- Mutant erythropoietin enhances white matter repair via the JAK2/STAT3 and C/EBPβ pathway in middle-aged mice following cerebral ischemia and reperfusion.
Wang R, Zhang S, Yang Z, Zheng Y, Yan F, Tao Z, Fan J, Zhao H, Han Z, Luo Y. Wang R, et al. Exp Neurol. 2021 Mar;337:113553. doi: 10.1016/j.expneurol.2020.113553. Epub 2020 Dec 9. Exp Neurol. 2021. PMID: 33309747 - Inhibition of CD147 improves oligodendrogenesis and promotes white matter integrity and functional recovery in mice after ischemic stroke.
Liu S, Jin R, Xiao AY, Zhong W, Li G. Liu S, et al. Brain Behav Immun. 2019 Nov;82:13-24. doi: 10.1016/j.bbi.2019.07.027. Epub 2019 Jul 26. Brain Behav Immun. 2019. PMID: 31356925 Free PMC article. - Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone.
Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Luo Y, et al. J Neurochem. 2006 Apr;97(2):435-48. doi: 10.1111/j.1471-4159.2006.03758.x. Epub 2006 Mar 15. J Neurochem. 2006. PMID: 16539667 - NG2-glia cell proliferation and differentiation by glial growth factor 2 (GGF2), a strategy to promote functional recovery after ischemic stroke.
Li F, Liu WC, Wang Q, Sun Y, Wang H, Jin X. Li F, et al. Biochem Pharmacol. 2020 Jan;171:113720. doi: 10.1016/j.bcp.2019.113720. Epub 2019 Nov 18. Biochem Pharmacol. 2020. PMID: 31751533 Review. - Subcortical ischemic vascular disease: Roles of oligodendrocyte function in experimental models of subcortical white-matter injury.
Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K. Shindo A, et al. J Cereb Blood Flow Metab. 2016 Jan;36(1):187-98. doi: 10.1038/jcbfm.2015.80. J Cereb Blood Flow Metab. 2016. PMID: 25920960 Free PMC article. Review.
Cited by
- Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage.
Guo Y, Dai W, Zheng Y, Qiao W, Chen W, Peng L, Zhou H, Zhao T, Liu H, Zheng F, Sun P. Guo Y, et al. Molecules. 2022 Oct 20;27(20):7080. doi: 10.3390/molecules27207080. Molecules. 2022. PMID: 36296682 Free PMC article. Review. - Emerging Targets for Modulation of Immune Response and Inflammation in Stroke.
Thapa K, Shivam K, Khan H, Kaur A, Dua K, Singh S, Singh TG. Thapa K, et al. Neurochem Res. 2023 Jun;48(6):1663-1690. doi: 10.1007/s11064-023-03875-2. Epub 2023 Feb 10. Neurochem Res. 2023. PMID: 36763312 Review. - How cytosolic compartments play safeguard functions against neuroinflammation and cell death in cerebral ischemia.
Ryan F, Khoshnam SE, Khodagholi F, Ashabi G, Ahmadiani A. Ryan F, et al. Metab Brain Dis. 2021 Oct;36(7):1445-1467. doi: 10.1007/s11011-021-00770-z. Epub 2021 Jun 26. Metab Brain Dis. 2021. PMID: 34173922 Review. - Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation.
Villapol S. Villapol S. Cell Mol Neurobiol. 2018 Jan;38(1):121-132. doi: 10.1007/s10571-017-0554-5. Epub 2017 Oct 3. Cell Mol Neurobiol. 2018. PMID: 28975471 Free PMC article. Review. - Secondary White Matter Injury Mediated by Neuroinflammation after Intracerebral Hemorrhage and Promising Therapeutic Strategies of Targeting the NLRP3 Inflammasome.
Xiao L, Wang M, Shi Y, Xu Y, Gao Y, Zhang W, Wu Y, Deng H, Pan W, Wang W, Sun H. Xiao L, et al. Curr Neuropharmacol. 2023;21(3):669-686. doi: 10.2174/1570159X20666220830115018. Curr Neuropharmacol. 2023. PMID: 36043798 Free PMC article. Review.
References
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. discussion 1647. - PubMed
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:263–274. - PubMed
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, et al. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke. 2005;36:2742–2744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS036736/NS/NINDS NIH HHS/United States
- R01 NS092618/NS/NINDS NIH HHS/United States
- NS092618/NS/NINDS NIH HHS/United States
- R01 NS089534/NS/NINDS NIH HHS/United States
- R01 NS095671/NS/NINDS NIH HHS/United States
- NS095671/NS/NINDS NIH HHS/United States
- I01 RX000420/RX/RRD VA/United States
- NS045048/NS/NINDS NIH HHS/United States
- NS089534/NS/NINDS NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources