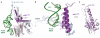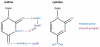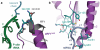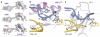Structural basis for stop codon recognition in eukaryotes - PubMed (original) (raw)
. 2015 Aug 27;524(7566):493-496.
doi: 10.1038/nature14896. Epub 2015 Aug 5.
Affiliations
- PMID: 26245381
- PMCID: PMC4591471
- DOI: 10.1038/nature14896
Structural basis for stop codon recognition in eukaryotes
Alan Brown et al. Nature. 2015.
Abstract
Termination of protein synthesis occurs when a translating ribosome encounters one of three universally conserved stop codons: UAA, UAG or UGA. Release factors recognize stop codons in the ribosomal A-site to mediate release of the nascent chain and recycling of the ribosome. Bacteria decode stop codons using two separate release factors with differing specificities for the second and third bases. By contrast, eukaryotes rely on an evolutionarily unrelated omnipotent release factor (eRF1) to recognize all three stop codons. The molecular basis of eRF1 discrimination for stop codons over sense codons is not known. Here we present cryo-electron microscopy (cryo-EM) structures at 3.5-3.8 Å resolution of mammalian ribosomal complexes containing eRF1 interacting with each of the three stop codons in the A-site. Binding of eRF1 flips nucleotide A1825 of 18S ribosomal RNA so that it stacks on the second and third stop codon bases. This configuration pulls the fourth position base into the A-site, where it is stabilized by stacking against G626 of 18S rRNA. Thus, eRF1 exploits two rRNA nucleotides also used during transfer RNA selection to drive messenger RNA compaction. In this compacted mRNA conformation, stop codons are favoured by a hydrogen-bonding network formed between rRNA and essential eRF1 residues that constrains the identity of the bases. These results provide a molecular framework for eukaryotic stop codon recognition and have implications for future studies on the mechanisms of canonical and premature translation termination.
Figures
**Extended Data Figure 1.. eRF1AAQ stalls ribosomes at stop codons
a, Line diagrams of mRNA encoding nascent chain (NC) substrates used in this study. The cytosolic portion of human Sec61β (residues 1-68, orange) was modified to contain an N-terminal 3× Flag tag (green) for affinity purification and the autonomously-folding villin headpiece (VHP, blue) domain. The three stop codons were individually inserted after Val68 of Sec61β to generate substrates for eRF1AAQ-mediated stalling, or the mRNA was truncated after the same residue to generate an independently-stalling substrate. b, In vitro translation reactions of NC-stop substrates containing the indicated stop codon (see panel a) in the presence of 35S-methionine without or with excess eRF1WT or eRF1AAQ. Reactions were for 25 min at 32°C and directly analyzed by SDS-PAGE and auto-radiography. The terminated (NC) and tRNA-associated (NC-tRNA) nascent chain products are indicated. Addition of eRF1AAQ selectively prevents peptide hydrolysis when the stop codon is reached. c, Anti-Flag affinity purifications of ribosome-nascent chains (RNCs) stalled either by mRNA truncation or at the UAA stop codon with eRF1AAQ (see panel a) were immunoblotted for the splitting factors Hbs1 and ABCE1. The different amounts of Hbs1 and ABCE1 co-purified despite identical nascent chain sequences in each RNC complex suggest that eRF1AAQ selectively traps ABCE1 on pre-termination complexes. d, SDS-PAGE and Coomassie staining of affinity-purified eRF1AAQ-stalled ribosome-nascent chains containing the UGA stop codon utilised for cryo-EM analysis. Bands corresponding to ribosomal proteins, ABCE1, and eRF1AAQ, which were verified by immunoblotting and mass spectrometry (data not shown), are indicated.
**Extended Data Figure 2.. In silico 3D classification scheme for cryo-EM datasets
Particles extracted from automated particle picking in Relion were subjected to 2D classification. Non-ribosomal particles were discarded and the remaining particles were combined for a 3D refinement. The resulting map was used as a reference for 3D classification, which typically isolated 5 distinct classes of ribosomal complexes with the indicated distributions. Classes containing 80S ribosomes with canonical P- and E-site tRNAs and weak factor density in the A site (~40%) were combined and subjected to another round of 3D classification for A site occupancy. Approximately 1/3 of this population contained strong density for eRF1AAQ and ABCE1. These particles were combined for subsequent 3D refinement and movie processing. All four datasets (two for the UAA stop codon and one each for the UAG and UGA stop codons) were processed similarly. The eRF1AAQ-ABCE1-containing particles of the two UAA datasets after the two rounds of classification were combined for refinement to yield the final map.
**Extended Data Figure 3.. Quality of maps and models
a, Fourier shell correlation (FSC) curves for the EM maps of each termination complex containing the indicated stop codon. b, Isolated eRF1AAQ-ABCE1 density from the UAA termination complex map coloured by local resolution. c, Fit of models to maps. FSC curves calculated between the refined model and the final map (black), and with the self- (blue) and cross-validated (magenta) correlations for each stop codon complex. The EM map of each termination complex coloured by local resolution (as in panel b) is displayed next to the corresponding curves.
**Extended Data Figure 4.. eRF1AAQ interactions within the termination complex
a, Comparison of ribosome-bound eRF1AAQ (coloured by domain) with the crystal structure of eRF1 (PDB ID: 1DT9, grey) superposed on the C domain. Both the N and M domains of eRF1 rotate upon stop codon recognition on the ribosome. The P-site tRNA (green) and nascent chain (teal) are shown for orientation. b, Interaction of helix α2 of the N domain of eRF1AAQ (purple) with the anticodon stem loop (ASL) of the P-site tRNA (green). c, Superposition of the eRF1AAQ M domain (purple) with the eRF1 crystal structure (PDB ID: 1DT9) showing a 10 Å movement of the GGQ-loop to accommodate within the peptidyl transferase centre.
**Extended Data Figure 5.. Examples of map densities
a, Density (from the UAG-containing termination complex) for the nascent chain (teal) attached to the CCA end of the P-site tRNA (green) is of sufficient resolution to model the defined sequence of the C-terminal end of the programmed nascent chain. This provides additional verification that the termination complexes are stalled at Val68 of Sec61β (human numbering) with the stop codon in the A site (see also Extended Data Fig. 1a). A stacking interaction between an aromatic residue of the nascent chain and U4555 (blue) lining the ribosomal exit tunnel can also be observed. b, Densities for the interactions between the UAG stop codon (grey), a portion of h44 of 18S rRNA (yellow) and the YxCxxxF and NIKS motifs of eRF1AAQ (purple). The invariant isoleucine of the NIKS motif provides a hydrophobic base for the stacking of the +2 and +3 bases of the stop codon with A1825. Unlike the tyrosine and cysteine residues of the YxCxxxF motif, the phenylalanine does not contribute to stop codon recognition, but to the hydrophobic packing of the eRF1 N domain.
**Extended Data Figure 6.. Hydrogen bonds specify for uridine at the +1 position
Chemical diagrams of uridine and cytidine with hydrogen bond donors (blue) and acceptors (magenta) indicated. Two of the three hydrogen bonds that uridine forms with Asn61 and Lys63 of the NIKS motif of eRF1AAQ (purple) are not possible with cytidine (see also Fig. 4a).
Figure 1. Overall structure of a eukaryotic translation termination complex
a, Overview of the structure of an eRF1AAQ-stalled mammalian ribosome-nascent chain complex containing the UAG stop codon showing the 40S and 60S ribosomal subunits, E- (yellow) and P-site (green) tRNAs, eRF1AAQ (purple) occupying the A site, and ABCE1 (blue) occupying the GTPase centre. b, Close-up view of eRF1AAQ coloured by domain (N, M, C) with the GGQ, NIKS and YxCxxxF motifs highlighted (pink). Also shown are the P-site tRNA (green), nascent polypeptide (teal), the mRNA containing the UAG stop codon (slate), and ABCE1 (blue) with its iron-sulfur clusters (orange/yellow) and nucleotide binding sites (gray) indicated.
Figure 2. Conformation of essential eRF1 motifs
a, Conformation of the GGQ-loop (teal) of eRF1AAQ (purple) within the peptidyl transferase centre. The AAQ tripeptide, positioned next to the CCA end of the P-site tRNA (green), closely resembles the conformation adopted by GGQ of a bacterial release factor (RF1, grey) bound to the ribosome. b, Positions of NIKS, YxCxxxF, and GTS (teal) motifs in the N domain of eRF1AAQ (purple) relative to the mRNA (slate). The positions of the stop codon (+1 to +3) and the following base (+4) are indicated.
Figure 3. Stop codon configuration in the eukaryotic decoding centre
a, EM map densities of the mRNA in the termination complexes containing the UAA, UAG and UGA stop codons reveal that they adopt the same compacted conformation. The ValGUU codon in the P site and the stop codon (+1 to +3) and following (+4) bases in the A site (purple) are indicated. b, The core termination signal recognised by eRF1AAQ (purple) is formed by four mRNA bases (+1 to +4, slate) that occupy the A site. Bases +2 and +3 stack on A1825, which is flipped out of helix 44 (h44), and base +4 on G626 of 18S rRNA (yellow). c, In bacteria, RF1 (grey) recognises a more extended stop codon configuration where the +3 base stacks on G530 (the equivalent of G626) of 16S rRNA.
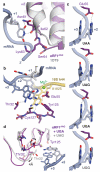
Figure 4. Molecular basis of stop codon recognition by eRF1
a, The conformation of the NIKS motif (purple) at the end of helix α2 of ribosome-bound eRF1AAQ compared to the eRF1 crystal structure (PDB ID: 1DT9, light grey) allows it to form hydrogen bonds with the uridine in the +1 position of stop codons (slate; see also Extended Data Fig. 6). The hydroxyl groups of Ser64 and Cδ–hydroxylated (*) Lys63 help to position the NIKS motif by interacting with the mRNA phosphate backbone. b, Detailed interactions between the UAG stop codon (slate), eRF1AAQ (purple), and A1825 (yellow), depicting (i) stacking of the +2 and +3 bases with A1825, (ii) stabilisation of the flipped out position of A1825 by hydrogen bonds with the main chain of Cys127, (iii) a hydrogen bonding network involving Thr32, Glu55 and Tyr125 that specifies stop codon selectivity, and (iv) coordination of a Mg2+ ion (green) by the +2 adenosine. c, Model for stop codon discrimination by eRF1. Stacking of the +2 and +3 bases (slate) and possible hydrogen bonding interactions with Glu55 of eRF1 (purple) are shown for the three stop codons (UAA, UAG, UGA) and the sense codon UGG, which codes for tryptophan. d, The UGA stop codon induces a conformational change in the YxCxxxF and GTS motifs of eRF1AAQ (purple) compared to UAG- (white) and UAA-bound eRF1AAQ and eRF1 crystal structures.
Similar articles
- Structure-Based Energetics of Stop Codon Recognition by Eukaryotic Release Factor.
Kumar A, Basu D, Satpati P. Kumar A, et al. J Chem Inf Model. 2017 Sep 25;57(9):2321-2328. doi: 10.1021/acs.jcim.7b00340. Epub 2017 Sep 6. J Chem Inf Model. 2017. PMID: 28825483 - Stop codons and UGG promote efficient binding of the polypeptide release factor eRF1 to the ribosomal A site.
Chavatte L, Frolova L, Laugâa P, Kisselev L, Favre A. Chavatte L, et al. J Mol Biol. 2003 Aug 22;331(4):745-58. doi: 10.1016/s0022-2836(03)00813-1. J Mol Biol. 2003. PMID: 12909007 - Mechanism of premature translation termination on a sense codon.
Svidritskiy E, Demo G, Korostelev AA. Svidritskiy E, et al. J Biol Chem. 2018 Aug 10;293(32):12472-12479. doi: 10.1074/jbc.AW118.003232. Epub 2018 Jun 25. J Biol Chem. 2018. PMID: 29941456 Free PMC article. - Molecular recognition and catalysis in translation termination complexes.
Klaholz BP. Klaholz BP. Trends Biochem Sci. 2011 May;36(5):282-92. doi: 10.1016/j.tibs.2011.02.001. Epub 2011 Mar 17. Trends Biochem Sci. 2011. PMID: 21420300 Review. - Structural aspects of translation termination on the ribosome.
Korostelev AA. Korostelev AA. RNA. 2011 Aug;17(8):1409-21. doi: 10.1261/rna.2733411. Epub 2011 Jun 23. RNA. 2011. PMID: 21700725 Free PMC article. Review.
Cited by
- mRNA-specific readthrough of nonsense codons by antisense oligonucleotides (R-ASOs).
Susorov D, Echeverria D, Khvorova A, Korostelev AA. Susorov D, et al. Nucleic Acids Res. 2024 Aug 27;52(15):8687-8701. doi: 10.1093/nar/gkae624. Nucleic Acids Res. 2024. PMID: 39011883 Free PMC article. - Termi-Luc: a versatile assay to monitor full-protein release from ribosomes.
Susorov D, Egri S, Korostelev AA. Susorov D, et al. RNA. 2020 Dec;26(12):2044-2050. doi: 10.1261/rna.076588.120. Epub 2020 Aug 14. RNA. 2020. PMID: 32817446 Free PMC article. - Receptor compaction and GTPase rearrangement drive SRP-mediated cotranslational protein translocation into the ER.
Lee JH, Jomaa A, Chung S, Hwang Fu YH, Qian R, Sun X, Hsieh HH, Chandrasekar S, Bi X, Mattei S, Boehringer D, Weiss S, Ban N, Shan SO. Lee JH, et al. Sci Adv. 2021 May 21;7(21):eabg0942. doi: 10.1126/sciadv.abg0942. Print 2021 May. Sci Adv. 2021. PMID: 34020957 Free PMC article. - The ribosomal stalk protein is crucial for the action of the conserved ATPase ABCE1.
Imai H, Abe T, Miyoshi T, Nishikawa SI, Ito K, Uchiumi T. Imai H, et al. Nucleic Acids Res. 2018 Sep 6;46(15):7820-7830. doi: 10.1093/nar/gky619. Nucleic Acids Res. 2018. PMID: 30010948 Free PMC article. - uS3/Rps3 controls fidelity of translation termination and programmed stop codon readthrough in co-operation with eIF3.
Poncová K, Wagner S, Jansen ME, Beznosková P, Gunišová S, Herrmannová A, Zeman J, Dong J, Valášek LS. Poncová K, et al. Nucleic Acids Res. 2019 Dec 2;47(21):11326-11343. doi: 10.1093/nar/gkz929. Nucleic Acids Res. 2019. PMID: 31642471 Free PMC article.
References
- Frolova L, et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994;372:701–703. - PubMed
- Jackson RJ, Hellen CUT, Pestova TV. Termination and post-termination events in eukaryotic translation. Adv Protein Chem Struct Biol. 2012;86:45–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 096570/WT_/Wellcome Trust/United Kingdom
- MC_U105184332/MRC_/Medical Research Council/United Kingdom
- MC_UP_A022_1007/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources