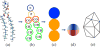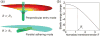Physical Principles of Nanoparticle Cellular Endocytosis - PubMed (original) (raw)
Review
Physical Principles of Nanoparticle Cellular Endocytosis
Sulin Zhang et al. ACS Nano. 2015.
Abstract
This review article focuses on the physiochemical mechanisms underlying nanoparticle uptake into cells. When nanoparticles are in close vicinity to a cell, the interactions between the nanoparticles and the cell membrane generate forces from different origins. This leads to the membrane wrapping of the nanoparticles followed by cellular uptake. This article discusses how the kinetics, energetics, and forces are related to these interactions and dependent on the size, shape, and stiffness of nanoparticles, the biomechanical properties of the cell membrane, as well as the local environment of the cells. The discussed fundamental principles of the physiochemical causes for nanoparticle-cell interaction may guide new studies of nanoparticle endocytosis and lead to better strategies to design nanoparticle-based approaches for biomedical applications.
Keywords: cellular uptake; coarse-grained model; endocytosis; ligand−receptor binding; membrane bending; membrane tension; nanomedicine; nanoparticles.
Conflict of interest statement
Conflict of Interest:
The authors declare no competing financial interest.
Figures
Figure 1
Possible internalization pathways of NPs.
Figure 2
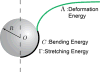
Membrane deformation energies when wrapping a NP.
Figure 3
(a) Schematic illustration of membrane wrapping of a single NP, driven by ligand–receptor binding. Wrapping depletes the receptors in the near vicinity of the NP, creating a receptor concentration gradient that drives the diffusion of the receptors from the remote region to the binding sites. (b) Interrelated effect of NP size and ligand density on the endocytic time of a NP. Reproduced from ref . Copyright 2010 American Physical Society.
Figure 4
Simultaneous entry of multiple NPs. (a) Schematics. (b) Phase diagram of cellular uptake in the space of particle size and ligand density. Reproduced from ref . Copyright 2010 American Physical Society.
Figure 5
Simulation models of lipids at different length scale. (a) All-atom model of the DMPC lipid molecules; (b and c) CG models, a 10-agent (b) and a 3-agent, (c) CG model; and a one-agent-thick membrane model (d). (e) Triangulated membrane model.,
Figure 6
(a–c) Simulation snapshots of the endocytic process of spherocylindrical NPs with different aspect ratios. For all the cases shown, R = 14 nm. (a) ρ = 1; (b) ρ = 1.5; (c) ρ = 2. Here, τ is a characteristic time scale of the model. Color-coded beads represent different coarse-grained constituents. Green, lipids; blue, receptors; yellow, ambient NP surface; red, ligands. (d). Shape effects on the endocytic time of NPs. Evolution of the areal wrapping fraction of NPs with the same radius (R = 10.0_σ_) but different aspect ratios. In the simulations, the spherocylindrical NPs are initially docked on the membrane with their long axes perpendicular to the membrane. Reprinted from ref . Copyright 2013 American Chemical Society.
Figure 7
(a) The bending energy profile for internalizing a spherocylindrical NP (ρ = 2) with different wrapping angles explains the laying-down-then-standing-up process. (red: θ = 0°; black: θ = 90°.). (b) Schematics of the laying-down-to-standing-up process. Images reprinted or adapted from ref . Copyright 2013 American Chemical Society.
Figure 8
(a) Two modes of interaction between a cell membrane and a nanotube. Reproduced from ref . Copyright 2014 American Chemical Society. (b) Elastic energy change as a function of the normalized membrane tension σ¯, where σ¯c=2π/5 is the critical point of transition between these two interaction modes.
Figure 9
(a) Schematic of typical wrapping states and (b and c) wrapping phase diagrams with respect to normalized adhesion energy and membrane tension σ¯ at different values of the rigidity ratio _B_L/B, where _B_L is the bending rigidity of the liposome; (b) 2D case; (c) 3D case. Dashed lines, boundaries between no wrapping and partial wrapping states; solid lines, boundaries between partial and full wrapping states. Adapted with permission from ref . Copyright 2011 American Physical Society.
Figure 10
Left: Cellular uptake of the fluorescent NPs by the cells on polyacrylamide substrates of varying stiffness. Cells were cultured on substrates for 12 h before loading the NPs. Images were taken after loading the NPs for 6 h. Right: The total fluorescence yield of individual cells on the polyacrylamide substrates of varying stiffness obtained by multiplying fluorescence per unit area by the projected cell area on a cell by cell basis. The difference between any two groups at any specified time point of measurement is statistically significant (p < 0.01 using Student t test). Reproduced from ref . Copyright 2013 American Chemical Society.
Figure 11
Left: Representative fluorescence images of SaOS-2 cells on various substrates with distinct surface topographies. Right: Cellular uptake of fluorescent NPs by cells on substrates of different surface topographies. The fluorescence intensities are normalized by the intensity on flat PMMA surface. **Significance at p < 0.01 between any two groups. Reproduced with permission from ref . Copyright 2015 Wiley & Sons, Inc.
Similar articles
- Understanding receptor-mediated endocytosis of elastic nanoparticles through coarse grained molecular dynamic simulation.
Shen Z , Ye H , Li Y . Shen Z , et al. Phys Chem Chem Phys. 2018 Jun 20;20(24):16372-16385. doi: 10.1039/c7cp08644j. Phys Chem Chem Phys. 2018. PMID: 29445792 - Mechanism of Coupling Nanoparticle Stiffness with Shape for Endocytosis: From Rodlike Penetration to Wormlike Wriggling.
Liu N, Becton M, Zhang L, Wang X. Liu N, et al. J Phys Chem B. 2020 Dec 10;124(49):11145-11156. doi: 10.1021/acs.jpcb.0c08089. Epub 2020 Nov 23. J Phys Chem B. 2020. PMID: 33226245 - A computational study of the influence of nanoparticle shape on clathrin-mediated endocytosis.
Li Y, Zhang M, Zhang Y, Niu X, Liu Z, Yue T, Zhang W. Li Y, et al. J Mater Chem B. 2023 Jul 12;11(27):6319-6334. doi: 10.1039/d3tb00322a. J Mater Chem B. 2023. PMID: 37232123 - Intracellular uptake, transport, and processing of gold nanostructures.
Chithrani DB. Chithrani DB. Mol Membr Biol. 2010 Oct;27(7):299-311. doi: 10.3109/09687688.2010.507787. Epub 2010 Oct 7. Mol Membr Biol. 2010. PMID: 20929337 Review. - Endocytosis at the nanoscale.
Canton I, Battaglia G. Canton I, et al. Chem Soc Rev. 2012 Apr 7;41(7):2718-39. doi: 10.1039/c2cs15309b. Epub 2012 Mar 5. Chem Soc Rev. 2012. PMID: 22389111 Review.
Cited by
- Ruthenium-initiated polymerization of lactide: a route to remarkable cellular uptake for photodynamic therapy of cancer.
Soliman N, McKenzie LK, Karges J, Bertrand E, Tharaud M, Jakubaszek M, Guérineau V, Goud B, Hollenstein M, Gasser G, Thomas CM. Soliman N, et al. Chem Sci. 2020 Jan 30;11(10):2657-2663. doi: 10.1039/c9sc05976h. Chem Sci. 2020. PMID: 34084324 Free PMC article. - Precision cardiac targeting: empowering curcumin therapy through smart exosome-mediated drug delivery in myocardial infarction.
Chen M, Wang S, Chen Y, Shen H, Chen L, Ding L, Tang Q, Yang Z, Chen W, Shen Z. Chen M, et al. Regen Biomater. 2023 Dec 13;11:rbad108. doi: 10.1093/rb/rbad108. eCollection 2024. Regen Biomater. 2023. PMID: 38223291 Free PMC article. - Combinatorial Delivery of miRNA-Nanoparticle Conjugates in Human Adipose Stem Cells for Amplified Osteogenesis.
Abu-Laban M, Hamal P, Arrizabalaga JH, Forghani A, Dikkumbura AS, Kumal RR, Haber LH, Hayes DJ. Abu-Laban M, et al. Small. 2019 Dec;15(50):e1902864. doi: 10.1002/smll.201902864. Epub 2019 Nov 14. Small. 2019. PMID: 31725198 Free PMC article. - Advanced Drug Delivery Systems for Renal Disorders.
Alallam B, Choukaife H, Seyam S, Lim V, Alfatama M. Alallam B, et al. Gels. 2023 Feb 1;9(2):115. doi: 10.3390/gels9020115. Gels. 2023. PMID: 36826285 Free PMC article. Review. - Unraveling the enigma: elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action.
Shardlow E, Mold M, Exley C. Shardlow E, et al. Allergy Asthma Clin Immunol. 2018 Nov 7;14:80. doi: 10.1186/s13223-018-0305-2. eCollection 2018. Allergy Asthma Clin Immunol. 2018. PMID: 30455719 Free PMC article. Review.
References
- Liu X, Dai Q, Austin L, Coutts J, Knowles G, Zou J, Chen H, Huo Q. A One-Step Homogeneous Immunoassay for Cancer Biomarker Detection Using Gold Nanoparticle Probes Coupled with Dynamic Light Scattering. J Am Chem Soc. 2008;130:2780–2782. - PubMed
- Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. Multiplexed Detection of Protein Cancer Markers with Biobarcoded Nanoparticle Probes. J Am Chem Soc. 2006;128:8378–8379. - PubMed
- Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In Vivo Tumor Targeting and Spectroscopic Detection with Surface-Enhanced Raman Nanoparticle Tags. Nat Biotechnol. 2008;26:83–90. - PubMed
- Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology Applications in Cancer. Annu Rev Biomed Eng. 2007;9:257–288. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 HL122902/HL/NHLBI NIH HHS/United States
- HHSN268201000043C/HL/NHLBI NIH HHS/United States
- PN2EY018244/EY/NEI NIH HHS/United States
- PN2 EY018244/EY/NEI NIH HHS/United States
- HHSN268201000043C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources