S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy - PubMed (original) (raw)
. 2015 Oct 9;117(9):793-803.
doi: 10.1161/CIRCRESAHA.115.307157. Epub 2015 Aug 10.
Patrick Y Sips 1, Shinichi Kai 1, Kotaro Kida 1, Kohei Ikeda 1, Shuichi Hirai 1, Kasra Moazzami 1, Pawina Jiramongkolchai 1, Donald B Bloch 1, Paschalis-Thomas Doulias 1, Antonis A Armoundas 1, Masao Kaneki 1, Harry Ischiropoulos 1, Evangelia Kranias 1, Kenneth D Bloch 1, Jonathan S Stamler 1, Fumito Ichinose 2
Affiliations
- PMID: 26259881
- PMCID: PMC4600453
- DOI: 10.1161/CIRCRESAHA.115.307157
S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy
Tomoya Irie et al. Circ Res. 2015.
Abstract
Rationale: The regulation of calcium (Ca(2+)) homeostasis by β-adrenergic receptor (βAR) activation provides the essential underpinnings of sympathetic regulation of myocardial function, as well as a basis for understanding molecular events that result in hypertrophic signaling and heart failure. Sympathetic stimulation of the βAR not only induces protein phosphorylation but also activates nitric oxide-dependent signaling, which modulates cardiac contractility. Nonetheless, the role of nitric oxide in βAR-dependent regulation of Ca(2+) handling has not yet been explicated fully.
Objective: To elucidate the role of protein S-nitrosylation, a major transducer of nitric oxide bioactivity, on βAR-dependent alterations in cardiomyocyte Ca(2+) handling and hypertrophy.
Methods and results: Using transgenic mice to titrate the levels of protein S-nitrosylation, we uncovered major roles for protein S-nitrosylation, in general, and for phospholamban and cardiac troponin C S-nitrosylation, in particular, in βAR-dependent regulation of Ca(2+) homeostasis. Notably, S-nitrosylation of phospholamban consequent upon βAR stimulation is necessary for the inhibitory pentamerization of phospholamban, which activates sarcoplasmic reticulum Ca(2+)-ATPase and increases cytosolic Ca(2+) transients. Coincident S-nitrosylation of cardiac troponin C decreases myocardial sensitivity to Ca(2+). During chronic adrenergic stimulation, global reductions in cellular S-nitrosylation mitigate hypertrophic signaling resulting from Ca(2+) overload.
Conclusions: S-Nitrosylation operates in concert with phosphorylation to regulate many cardiac Ca(2+)-handling proteins, including phospholamban and cardiac troponin C, thereby playing an essential and previously unrecognized role in cardiac Ca(2+) homeostasis. Manipulation of the S-nitrosylation level may prove therapeutic in heart failure.
Keywords: beta adrenergic; calcium; heart failure; myocardial contraction; nitric oxide; receptors.
© 2015 American Heart Association, Inc.
Figures
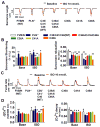
Figure 1. Enhanced denitrosylation alters the intracellular Ca2+ response to βAR stimulation
(A) Left, representative continuous recordings of sarcomere length and Ca2+ transients (Δ[Ca2+]) in isolated cardiomyocytes before and during infusion of ISO at 10 nmol/L. Right, representative traces of sarcomere length and Δ[Ca2+] after different doses of ISO in WT and GSNOR-Tg (TG) cardiomyocytes paced at 2 Hz. (B) Dose-dependent changes of sarcomere shortening and Δ[Ca2+] in response to ISO in isolated WT and TG cardiomyocytes. n=4 mice (13–18 cells per mouse) per group. (C) Calcineurin activity in isolated WT and TG hearts treated with or without ISO (10 μmol/l). n=5 mice per group. (D) Sarcomere shortening and Δ[Ca2+] in response to ISO at 10 nmol/L with or without Carboxy-PTIO (C-PTIO, 300 μmol/L) in isolated WT cardiomyocytes. n=4 mice (11–15 cells per mouse) per group. ##P<0.01 and ###P<0.001 (vs baseline), *P<0.05, **P<0.01, ***P<0.001.
Figure 2. Enhanced denitrosylation mitigates LV remodeling after chronic βAR stimulation
(A) Representative echocardiography images (Top), heart images (middle) and histological images of hematoxylin and eosin staining for cardiomyocyte area (upper histological image) and Masson’s Trichrome staining of interstitial fibrosis (lower histological image) at baseline and 28 days after starting ISO infusion for 14 days (ISO Day28, i.e., 14 days after the end of ISO infusion) in WT and TG mice. (B) Fractional shortening and left ventricular mass calculated by echocardiography at baseline, and 7, 14 and 28 days after the start of ISO infusion for 14 days in WT and TG mice. n=5–7 mice per group. (C) Heart weight/body weight ratio, heart weight/tibia length ratio, mean cardiomyocyte area in LV sections (average of 207±12 cells were measured from 20 fields per mouse), and interstitial fibrosis in LV section (11 fields per mouse) in WT and TG mice at baseline and 28 days after starting ISO infusion for 14 days. N=4–7 mice per group. (D) Representative western blots and summary data showing the amount of NFATc3, β-tubulin, and histone of purified nuclear and cytoplasmic fraction in WT and TG hearts. β-tubulin and histone were used as a loading control for purified nuclear and cytoplasmic fractions, respectively. n=4–6 mice per group. ##P<0.01 and ###P<0.001 (vs baseline), *P<0.05, **P<0.01, ***P<0.001.
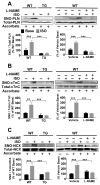
Figure 3. GSNOR overexpression or inhibition of NOS prevents isoproterenol-induced S-nitrosylation of Ca2+ handling proteins
Representative modified biotin switch assay blots of S-nitrosylated PLN (A), cTnC (B), and NCX (C) in GSNOR-Tg cardiomyocytes, and WT cardiomyocytes with or without L-NAME at baseline and after ISO treatment. n=5–6 mice per group. **P<0.01, ***P<0.001.
Figure 4. PLN S-nitrosylation is required for isoproterenol to induce PLN pentamerization and activate SERCA2a
(A) Representative tracings of SR Ca2+ load measurements in WT and GSNOR-Tg (TG) cardiomyocytes at baseline and after ISO stimulation (10, 100 and 1000 nmol/L). Dose-dependent changes of SR Ca2+ load, SR Ca2+ leak, and fractional Ca2+ release in WT and TG cardiomyocytes in response to ISO stimulation. n=18–25 cells per group. ##P<0.01, ###P<0.001 (vs baseline), **P<0.01, ***P<0.001. (B) Representative immunoblots showing levels of pentamer and monomer PLN in TG hearts, and WT hearts with or without carboxy-PTIO (C-PTIO) at baseline and after ISO treatment. Samples that were boiled and treated with dithiothreitol (DTT) are shown as all monomer control. Effects of ISO on PLN pentamer/monomer ratio calculated from immunoblots in TG and WT with and without carboxy-PTIO. Data were normalized to the levels in WT hearts at baseline. (C) Representative immunoblots and relative quantification of PLN phosphorylation at Ser16 and Thr16 versus total PLN in WT and TG cardiomyocytes without (Base) or with ISO treatment. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs. baseline.
Figure 5. S-nitrosylation of PLN at Cys36 and/or Cys41 is required for βAR-induced SERCA2a activation
(A) Representative tracings of sarcomere length of wild-type FVB/N transfected with empty virus (FVB/N), PLN−/− cardiomyocytes transfected with empty virus (PLN−/−), and PLN−/− cardiomyocytes expressing wild-type (C36/C41/C46 WT) or mutant PLN with or without ISO treatment. (B) Summary of percent sarcomere shortening and relaxation rate. N=11–19. (C) Representative Ca2+ transient tracing and (D) summary of Δ[Ca2+] and Ca2+ decay rate. N=11–19 *P<0.01 (vs baseline), #P<0.01 and ##P<0.001 (vs FVB/N).
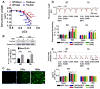
Figure 6. De-nitrosylation of cTnC increases myocardial Ca2+ sensitivity during βAR activation
(A) Myofilament Ca2+ sensitivity in Triton-permeabilized WT and GSNOR-Tg (TG) cardiomyocytes at baseline and after ISO challenge (10 nmol/L). (B) Ser22,23 phosphorylation of cTnI in WT and TG cardiomyocytes without (base) or with ISO. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs. baseline. (C) Representative photomicrographs of cardiomyocytes culture before and 24 and 48 hours after infection with Ad.cTnC.EGFP. (D) Representative sarcomere length tracings and summary of percent sarcomere shortening in WT (n=20–28 cells per group) and TG (n=24–36 cells per group) cardiomyocytes expressing WT or mutant cTnC with or without ISO treatment. (E) Representative Δ[Ca2+] tracing and summary of Δ[Ca2+] of WT and TG cardiomyocytes expressing WT or mutant cTnC with or without ISO treatment. **P<0.01, ***P<0.001 (* vs each baseline), and ###P<0.001 (# vs other data points).
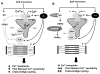
Figure 7. Hypothetical impact of protein SNO on Ca2+ handling in βAR stimulation
(A) Normal cardiomyocytes with intact PKA-dependent phosphorylation and NO-dependent SNO. (B) Cardiomyocytes with GSNOR-Tg or treated with carboxy-PTIO (C-PTIO) with impaired SNO.
Comment in
- Adrenergic Fight-or-Flight: S-NO Falls on PKA Targets.
Bers DM. Bers DM. Circ Res. 2015 Oct 9;117(9):747-9. doi: 10.1161/CIRCRESAHA.115.307397. Circ Res. 2015. PMID: 26450885 Free PMC article.
Similar articles
- Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart.
Wang G, Zhu X, Xie W, Han P, Li K, Sun Z, Wang Y, Chen C, Song R, Cao C, Zhang J, Wu C, Liu J, Cheng H. Wang G, et al. Circ Res. 2010 Feb 5;106(2):317-27. doi: 10.1161/CIRCRESAHA.109.208272. Epub 2009 Nov 19. Circ Res. 2010. PMID: 19926875 - Balanced changes in Ca buffering by SERCA and troponin contribute to Ca handling during β-adrenergic stimulation in cardiac myocytes.
Briston SJ, Dibb KM, Solaro RJ, Eisner DA, Trafford AW. Briston SJ, et al. Cardiovasc Res. 2014 Nov 1;104(2):347-54. doi: 10.1093/cvr/cvu201. Epub 2014 Sep 2. Cardiovasc Res. 2014. PMID: 25183792 Free PMC article. - Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice.
Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW 2nd, Walsh RA, Kranias EG. Kadambi VJ, et al. J Clin Invest. 1996 Jan 15;97(2):533-9. doi: 10.1172/JCI118446. J Clin Invest. 1996. PMID: 8567978 Free PMC article. - Targeting myocardial beta-adrenergic receptor signaling and calcium cycling for heart failure gene therapy.
Pleger ST, Boucher M, Most P, Koch WJ. Pleger ST, et al. J Card Fail. 2007 Jun;13(5):401-14. doi: 10.1016/j.cardfail.2007.01.003. J Card Fail. 2007. PMID: 17602988 Review. - cAMP-mediated signal transduction and sarcoplasmic reticulum function in heart failure.
Movsesian MA. Movsesian MA. Ann N Y Acad Sci. 1998 Sep 16;853:231-9. doi: 10.1111/j.1749-6632.1998.tb08271.x. Ann N Y Acad Sci. 1998. PMID: 10603951 Review.
Cited by
- Nitric Oxide Synthase 1 Modulates Basal and β-Adrenergic-Stimulated Contractility by Rapid and Reversible Redox-Dependent S-Nitrosylation of the Heart.
Vielma AZ, León L, Fernández IC, González DR, Boric MP. Vielma AZ, et al. PLoS One. 2016 Aug 16;11(8):e0160813. doi: 10.1371/journal.pone.0160813. eCollection 2016. PLoS One. 2016. PMID: 27529477 Free PMC article. - Facilitating Nitrite-Derived S-Nitrosothiol Formation in the Upper Gastrointestinal Tract in the Therapy of Cardiovascular Diseases.
Silva-Cunha M, Lacchini R, Tanus-Santos JE. Silva-Cunha M, et al. Antioxidants (Basel). 2024 Jun 4;13(6):691. doi: 10.3390/antiox13060691. Antioxidants (Basel). 2024. PMID: 38929130 Free PMC article. Review. - Mesenchymal stem cells suppress cardiac alternans by activation of PI3K mediated nitroso-redox pathway.
Sattayaprasert P, Nassal DM, Wan X, Deschenes I, Laurita KR. Sattayaprasert P, et al. J Mol Cell Cardiol. 2016 Sep;98:138-45. doi: 10.1016/j.yjmcc.2016.05.014. Epub 2016 May 26. J Mol Cell Cardiol. 2016. PMID: 27238412 Free PMC article. - An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System.
Wu D, Hu Q, Zhu D. Wu D, et al. Oxid Med Cell Longev. 2018 Sep 9;2018:4579140. doi: 10.1155/2018/4579140. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30271527 Free PMC article. Review. - Hypoxia Rescues Frataxin Loss by Restoring Iron Sulfur Cluster Biogenesis.
Ast T, Meisel JD, Patra S, Wang H, Grange RMH, Kim SH, Calvo SE, Orefice LL, Nagashima F, Ichinose F, Zapol WM, Ruvkun G, Barondeau DP, Mootha VK. Ast T, et al. Cell. 2019 May 30;177(6):1507-1521.e16. doi: 10.1016/j.cell.2019.03.045. Epub 2019 Apr 25. Cell. 2019. PMID: 31031004 Free PMC article.
References
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
- Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin i in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL26057/HL/NHLBI NIH HHS/United States
- R37 HL026057/HL/NHLBI NIH HHS/United States
- R01 HL026057/HL/NHLBI NIH HHS/United States
- R01 HL054926/HL/NHLBI NIH HHS/United States
- P01 HL075443/HL/NHLBI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- HL110378/HL/NHLBI NIH HHS/United States
- HL075443/HL/NHLBI NIH HHS/United States
- R01 HL064018/HL/NHLBI NIH HHS/United States
- HL54926/HL/NHLBI NIH HHS/United States
- R01 HL110378/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous