Frontiers in research on biodiversity and disease - PubMed (original) (raw)
Review
. 2015 Oct;18(10):1119-33.
doi: 10.1111/ele.12479. Epub 2015 Aug 10.
Affiliations
- PMID: 26261049
- PMCID: PMC4860816
- DOI: 10.1111/ele.12479
Review
Frontiers in research on biodiversity and disease
Pieter T J Johnson et al. Ecol Lett. 2015 Oct.
Abstract
Global losses of biodiversity have galvanised efforts to understand how changes to communities affect ecological processes, including transmission of infectious pathogens. Here, we review recent research on diversity-disease relationships and identify future priorities. Growing evidence from experimental, observational and modelling studies indicates that biodiversity changes alter infection for a range of pathogens and through diverse mechanisms. Drawing upon lessons from the community ecology of free-living organisms, we illustrate how recent advances from biodiversity research generally can provide necessary theoretical foundations, inform experimental designs, and guide future research at the interface between infectious disease risk and changing ecological communities. Dilution effects are expected when ecological communities are nested and interactions between the pathogen and the most competent host group(s) persist or increase as biodiversity declines. To move beyond polarising debates about the generality of diversity effects and develop a predictive framework, we emphasise the need to identify how the effects of diversity vary with temporal and spatial scale, to explore how realistic patterns of community assembly affect transmission, and to use experimental studies to consider mechanisms beyond simple changes in host richness, including shifts in trophic structure, functional diversity and symbiont composition.
Keywords: Amplification effect; biodiversity loss; biodiversity-ecosystem function; community ecology; dilution effect; disease ecology; symbiont.
© 2015 John Wiley & Sons Ltd/CNRS.
Figures
Figure 1
Mechanisms through which diversity can alter pathogen transmission or disease risk (_sensu_Keesing et al. 2006). (a) Decreases or (b) increases in the density of susceptible hosts. Higher plant diversity reduces host availability for fungal pathogens (Mitchell et al. 2002), whereas invasive brown trout provide a reservoir for Myxobolus cerebralis, the cause of whirling disease (Vincent 1996); (c) Decreases or (d) increases in the encounter rate between suitable hosts and parasites. Consumption of chytrid zoospores by predators reduced infection in amphibians (c) (Schmeller et al. 2014), whereas fish increased infections in Daphnia magna by altering their habitat use (d) (Decaestecker et al. 2002); Changes in the rates at which infected hosts die (e) or recover (f). In (e), coinfection by nematodes and bacteria increased mortality in African buffalo, likely lowering transmission (Ezenwa & Jolles 2015); in (f), healthy faecal bacteria reduced pathogenic infections in humans (Costello et al. 2012).
Figure 2
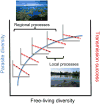
Diversity could have scale-dependent and even opposing effects on parasite colonisation, a regional process determining parasite diversity (shown in blue on the left _y_-axis), and pathogen transmission (shown red dashed lines on the right _y_-axis), a local scale process involving the capacity of a virulent pathogen to spread among hosts. Here, the positive effect of free-living richness on parasite richness begins to saturate with overall increases in diversity as a hypothetical community assembles in an increasingly substitutive (rather than additive) manner. Concurrently, the negative relationship between diversity and local pathogen transmission (i.e. a dilution effect) is strongest at low to intermediate levels of free-living richness, after which additional increases in richness have more modest effects on transmission. However, because parasites, vectors and hosts differ in mobility and range size, studies need to carefully consider the ‘ecological scale’ of the specific disease system under study.
Figure 3
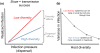
Contrasting the effects of additive and substitutive assembly on the relationship between diversity and community competence. Even if host species assembly order is inversely related to the species' competence (a), whether changes in diversity lead to a reduction in infection risk depends on how communities assemble. If communities assemble additively, then the total density (or biomass) of hosts will increase with species richness; substitutive assembly assumes a fixed carrying capacity for the community, such that increases in diversity are associated with replacement of established individuals to maintain a constant total density (b). This leads to very different patterns in the total competence of the community (the product of each species' abundance multiplied by its competence), which can strongly influence transmission and disease risk (depending on whether transmission is density- or frequency-dependent), even if the average competence per host (dashed line) decreases in both scenarios (c).
Figure 4
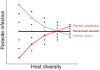
Hypothesised relationships among diversity, infection pressure and infection variance in a community. (a) Host infection is expected to be a function of both infection pressure (e.g. the density of infected vectors, reservoirs or infectious propagules) and the diversity of hosts; the interaction between these terms reflects pathogen transmission success, which here is shown to weaken at higher host diversity. Host diversity thus acts as a ‘niche-based’ filter upon dispersal pressure, which will also be affected by climate, resource availability and community structure. (b) Modelling studies suggest that host diversity will strongly affect the variance in infection or transmission (Mihaljevic et al. 2014). Regardless of transmission mode or assembly pattern, species-poor communities have higher variance in epidemic size (over time or space), whereas diverse communities exhibit lower variance, emphasising the importance of collecting sufficient data to explore infection responses and their temporal or spatial variance along diversity gradients.
Figure 5
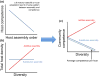
Hypothetical effects of random vs. realistic community structures on pathogen infection success and disease risk. Because the selection of a particular focal host group and of particular community permutations will influence the perceived diversity–disease relationship, it is important to consider the influence of realistic changes in community structure on total infection. If communities assemble randomly and there are no non-additive effects (e.g. complementarity), then diversity will have no relationship with average parasite infection (solid black line). If, however, if the order in which species assemble is deterministic and negatively related to community competence (i.e. competent species are replaced or ‘diluted’ by less competent hosts at higher diversity), then experiments designed based on ‘realistic’ patterns of community structure will show dilution effects (blue line). If assembly is deterministic but positively related to total community competence, amplification effects are predicted (red line).
Similar articles
- Null expectations for disease dynamics in shrinking habitat: dilution or amplification?
Faust CL, Dobson AP, Gottdenker N, Bloomfield LSP, McCallum HI, Gillespie TR, Diuk-Wasser M, Plowright RK. Faust CL, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Jun 5;372(1722):20160173. doi: 10.1098/rstb.2016.0173. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28438921 Free PMC article. - Integrating species traits into species pools.
Spasojevic MJ, Catano CP, LaManna JA, Myers JA. Spasojevic MJ, et al. Ecology. 2018 Jun;99(6):1265-1276. doi: 10.1002/ecy.2220. Ecology. 2018. PMID: 29569239 Review. - Trait-based ecology of terrestrial arthropods.
Wong MKL, Guénard B, Lewis OT. Wong MKL, et al. Biol Rev Camb Philos Soc. 2019 Jun;94(3):999-1022. doi: 10.1111/brv.12488. Epub 2018 Dec 13. Biol Rev Camb Philos Soc. 2019. PMID: 30548743 Free PMC article. Review. - Linking community and disease ecology: the impact of biodiversity on pathogen transmission.
Roche B, Dobson AP, Guégan JF, Rohani P. Roche B, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Oct 19;367(1604):2807-13. doi: 10.1098/rstb.2011.0364. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22966136 Free PMC article. - Drawing ecological inferences from coincident patterns of population- and community-level biodiversity.
Vellend M, Lajoie G, Bourret A, Múrria C, Kembel SW, Garant D. Vellend M, et al. Mol Ecol. 2014 Jun;23(12):2890-901. doi: 10.1111/mec.12756. Epub 2014 May 25. Mol Ecol. 2014. PMID: 24750409 Review.
Cited by
- Zoonotic host diversity increases in human-dominated ecosystems.
Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, Jones KE. Gibb R, et al. Nature. 2020 Aug;584(7821):398-402. doi: 10.1038/s41586-020-2562-8. Epub 2020 Aug 5. Nature. 2020. PMID: 32759999 - Can predators stabilize host-parasite interactions? Changes in aquatic predator identity alter amphibian responses and parasite abundance across life stages.
Strasburg M, Boone MD. Strasburg M, et al. Ecol Evol. 2022 Nov 15;12(11):e9512. doi: 10.1002/ece3.9512. eCollection 2022 Nov. Ecol Evol. 2022. PMID: 36407903 Free PMC article. - Declining ecosystem health and the dilution effect.
Khalil H, Ecke F, Evander M, Magnusson M, Hörnfeldt B. Khalil H, et al. Sci Rep. 2016 Aug 8;6:31314. doi: 10.1038/srep31314. Sci Rep. 2016. PMID: 27499001 Free PMC article. - Diverging effects of host density and richness across biological scales drive diversity-disease outcomes.
Johnson PTJ, Stewart Merrill TE, Dean AD, Fenton A. Johnson PTJ, et al. Nat Commun. 2024 Mar 2;15(1):1937. doi: 10.1038/s41467-024-46091-4. Nat Commun. 2024. PMID: 38431719 Free PMC article. - Measuring the shape of the biodiversity-disease relationship across systems reveals new findings and key gaps.
Halliday FW, Rohr JR. Halliday FW, et al. Nat Commun. 2019 Nov 6;10(1):5032. doi: 10.1038/s41467-019-13049-w. Nat Commun. 2019. PMID: 31695043 Free PMC article.
References
- Allan BF, Langerhans RB, Ryberg WA, Landesman WJ, Griffin NW, Katz RS, et al. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia. 2009;158:699–708. - PubMed
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9:467–484. - PubMed
- Araújo MB, Rozenfeld A. The geographic scaling of biotic interactions. Ecography. 2014;37:406–415.
- Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: having right or wrong neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical