High magnitude, in vitro, biaxial, cyclic tensile strain induces actin depolymerization in tendon cells - PubMed (original) (raw)
eCollection 2015 Apr-Jun.
Affiliations
- PMID: 26261792
- PMCID: PMC4496012
High magnitude, in vitro, biaxial, cyclic tensile strain induces actin depolymerization in tendon cells
Michael Lavagnino et al. Muscles Ligaments Tendons J. 2015.
Abstract
Background: the cytoskeleton is a dynamic arrangement of actin filaments that maintain cell shape and are vital in mediating the mechanobiological response of the cell.
Methods: to determine the cytoskeletal response to varying in vitro, biaxial stretch amplitudes, rat-tail tendon cells were paired into control and cyclically strained groups of 4.75, 9.5, or 12% strain at 1 Hz for 2 hours and the actin cytoskeleton stained. The cells were analyzed for actin staining intensity as a measure of relative depolymerization and for cell shape. Collagenase gene expression was measured in cells undergoing 12% cyclic strain at 1 Hz for 24 hours.
Results: there was no significant difference in the degree of actin staining intensity between the control group and cells strained at either 4.75 or 9.5%. However, cells strained at 12% demonstrated a significant decrease in actin staining intensity (depolymerization) compared to control cells, increased collagenase expression by 81%, and a clear shift towards a more rounded cell shape.
Conclusion: the results of this study demonstrate that the previously reported induction of collagenase activity associated with the application of high magnitude, in vitro, tensile strains may actually be a result of cytoskeletal depolymerization, which causes loss of tensional homeostasis and alteration of cell shape.
Keywords: actin intensity; cell shape; collagenase; mechanobiology; tendinopathy; under-stimulation.
Figures
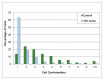
Figure 1
Histogram of percentage of cells within each cell conformation range for 15% strain as well as control cells. A cell conformation of 1 corresponds to a round cell while higher values correspond to more elongate cells.
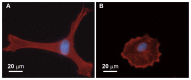
Figure 2
Representative photomicrographs of an elongate cell with intense fluorescence of actin stress fibers in the control group (A) and a round cell with less intense staining of the actin stress fibers in the 15% strain group (B).
Similar articles
- Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study.
Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Lavagnino M, et al. Connect Tissue Res. 2003;44(3-4):181-7. doi: 10.1080/03008200390215881. Connect Tissue Res. 2003. PMID: 14504039 - [Effects of different cyclic mechanical stretching loads on human tenocytic cytoskeleton in vitro].
Deng YS, Tang KL, Xie MM, Cao HH, Chen L, Chang DH, Dong SW, Tao X, Li H, Yang HF, Xu JZ. Deng YS, et al. Zhonghua Yi Xue Za Zhi. 2011 Jul 5;91(25):1780-5. Zhonghua Yi Xue Za Zhi. 2011. PMID: 22093739 Chinese. - Re-establishment of cytoskeletal tensional homeostasis in lax tendons occurs through an actin-mediated cellular contraction of the extracellular matrix.
Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP. Gardner K, et al. J Orthop Res. 2012 Nov;30(11):1695-701. doi: 10.1002/jor.22131. Epub 2012 Apr 19. J Orthop Res. 2012. PMID: 22517354 - The 1996 Lindberg Award. Calcium antagonists alter cell shape and induce procollagenase synthesis in keloid and normal human dermal fibroblasts.
Doong H, Dissanayake S, Gowrishankar TR, LaBarbera MC, Lee RC. Doong H, et al. J Burn Care Rehabil. 1996 Nov-Dec;17(6 Pt 1):497-514. J Burn Care Rehabil. 1996. PMID: 8951536 Review. - The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells?
Arnoczky SP, Lavagnino M, Egerbacher M. Arnoczky SP, et al. Int J Exp Pathol. 2007 Aug;88(4):217-26. doi: 10.1111/j.1365-2613.2007.00548.x. Int J Exp Pathol. 2007. PMID: 17696902 Free PMC article. Review.
Cited by
- Gene expression profiling of the masticatory muscle tendons and Achilles tendons under tensile strain in the Japanese macaque Macaca fuscata.
Ito K, Go Y, Tatsumoto S, Usui C, Mizuno Y, Ikami E, Isozaki Y, Usui M, Kajihara T, Yoda T, Inoue KI, Takada M, Sato T. Ito K, et al. PLoS One. 2023 Jan 19;18(1):e0280649. doi: 10.1371/journal.pone.0280649. eCollection 2023. PLoS One. 2023. PMID: 36656905 Free PMC article. - Stress deprivation of tendon explants or Tpm3.1 inhibition in tendon cells reduces F-actin to promote a tendinosis-like phenotype.
Inguito KL, Schofield MM, Faghri AD, Bloom ET, Heino M, West VC, Ebron KMM, Elliott DM, Parreno J. Inguito KL, et al. Mol Biol Cell. 2022 Dec 1;33(14):ar141. doi: 10.1091/mbc.E22-02-0067. Epub 2022 Sep 21. Mol Biol Cell. 2022. PMID: 36129771 Free PMC article. - Focal adhesion kinase regulates tendon cell mechanoresponse and physiological tendon development.
Leahy TP, Chenna SS, Soslowsky LJ, Dyment NA. Leahy TP, et al. FASEB J. 2024 Sep;38(17):e70050. doi: 10.1096/fj.202400151R. FASEB J. 2024. PMID: 39259535 - Hypoxia inhibits primary cilia formation and reduces cell-mediated contraction in stress-deprived rat tail tendon fascicles.
Lavagnino M, Oslapas AN, Gardner KL, Arnoczky SP. Lavagnino M, et al. Muscles Ligaments Tendons J. 2016 Sep 17;6(2):193-197. doi: 10.11138/mltj/2016.6.2.193. eCollection 2016 Apr-Jun. Muscles Ligaments Tendons J. 2016. PMID: 27900292 Free PMC article.
References
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;0:232–239. - PubMed
- Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35. - PubMed
- Jozsa LG, Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997.
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med Sci Sports Exerc. 1993;25:603–607. - PubMed
LinkOut - more resources
Full Text Sources