Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific Antibodies - PubMed (original) (raw)
Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific Antibodies
Cristina d'Abramo et al. PLoS One. 2015.
Abstract
Recent work from our lab and few others have strongly suggested that immunotherapy could be an effective means of preventing the development of tau accumulation in JNPL3 transgenic mice, carrying the human P301L mutation. The aim of this study was to test the efficacy of a variety of specific tau monoclonal antibodies in JNPL3. Starting at 3 months of age, mice were treated for 4 months with weekly intraperitoneal injections of saline or purified tau monoclonal antibodies (10 mg/Kg) different in specificity for pathological tau: CP13 (pSer202), RZ3 (pThr231) and PG5 (pSer409). As expected, not all the antibodies tested showed efficacy at preventing the development of tau pathology at the described dose, with some of them even worsening the pathological scenario. Only by targeting the pSer202 epitope with CP13 was a conspicuous reduction of insoluble or soluble tau in cortex and hindbrain obtained. Here we report about the importance of screening in vivo multiple tau antibodies in order to select the antibodies to direct into future clinical studies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
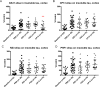
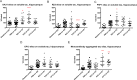
Fig 1. Effect of immunotherapy on insoluble tau in cortex.
Mice were treated with different antibodies from 3 to 7 months of age. Baseline (3 months) and PBS cohorts were included in the study. (A) After 4 months of treatment CP13 significantly decreases insoluble tau in cortex (**p = 0.0073). (B-D) None of the other antibodies exert any effect on insoluble tau in cortex. Results are expressed as % PBS control.
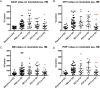
Fig 2. Effect of immunotherapy on insoluble tau in hindbrain.
(A) After 4 months of treatment no effect is evident on total insoluble tau, while (B) CP13 significantly decreases insoluble pSer202 tau in hindbrain (***p = 0.0001), together with (C) pThr231 (*p = 0.0258). Results are expressed as % PBS control.
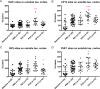
Fig 3. Effect of immunotherapy on soluble tau in cortex.
(A) PG5 increases the accumulation of soluble tau (**p = 0.0058) in cortex. (B,D) Phosphorylations at Ser202 and Ser396-404 are significantly higher than the PBS group when injecting PG5 (***p = 0.0007 and *p = 0.0155 respectively). (C) RZ3 ELISA detecting pThr231 shows lower phosphorylation when treating animals with CP13 (*p = 0.0124). Results are expressed as % PBS control.
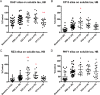
Fig 4. Effect of immunotherapy on soluble tau in hindbrain.
(A) animals injected with CP13 show decreased soluble tau levels in hindbrain (**p = 0.0039). (B,C) RZ3 increases phosphorylation at both Ser202 (*p = 0.0394) and Ser231 (**p = 0.0049), while PG5 significantly augments pThr231 (*p = 0.0236). No effect has been reported on Ser396-404 (D). Results are expressed as % PBS control.
Fig 5. Effect of immunotherapy on soluble and on aggregated tau in hippocampal fractions.
(A) PG5 and RZ3 increase soluble tau levels (***p = 0.0004 and p = 0.0317 respectively). (B) Phosphorylation at Thr231 on soluble tau is lowered by PG5 (***p<0.0001), while (C) pSer396-404 is increased when injecting RZ3 (*p = 0.0427). (E) The monoantibody ELISA to detect aggregated tau in the hippocampal fraction reveals increased levels of aggregated tau after injecting PG5 (**p = 0.0069) or CP13 (**p = 0.0049). Results are expressed as % PBS control.
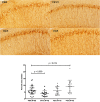
Fig 6. CP13 immunocytochemistry.
Representative stained sections from each treatment group are shown. Images as shown were analyzed with ImageJ for percent area stained. Each data point represents the mean value obtained from at least two sections per mouse. The area stained is significantly lower from CP13 treated mice (p < 0.009) and significantly higher in PG5 treated mice (p < 0.019).
Similar articles
- Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain.
Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Boutajangout A, et al. J Neurochem. 2011 Aug;118(4):658-67. doi: 10.1111/j.1471-4159.2011.07337.x. Epub 2011 Jul 1. J Neurochem. 2011. PMID: 21644996 Free PMC article. - Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice.
Vitale F, Giliberto L, Ruiz S, Steslow K, Marambaud P, d'Abramo C. Vitale F, et al. Acta Neuropathol Commun. 2018 Aug 22;6(1):82. doi: 10.1186/s40478-018-0585-2. Acta Neuropathol Commun. 2018. PMID: 30134961 Free PMC article. - Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity.
d'Abramo C, Acker CM, Jimenez HT, Davies P. d'Abramo C, et al. PLoS One. 2013 Apr 29;8(4):e62402. doi: 10.1371/journal.pone.0062402. Print 2013. PLoS One. 2013. PMID: 23638068 Free PMC article. - Immunotherapy for tauopathies.
Gu J, Sigurdsson EM. Gu J, et al. J Mol Neurosci. 2011 Nov;45(3):690-5. doi: 10.1007/s12031-011-9576-5. Epub 2011 Jul 8. J Mol Neurosci. 2011. PMID: 21739165 Free PMC article. Review. - Tau Immunotherapy.
Sigurdsson EM. Sigurdsson EM. Neurodegener Dis. 2016;16(1-2):34-8. doi: 10.1159/000440842. Epub 2015 Nov 10. Neurodegener Dis. 2016. PMID: 26551002 Free PMC article. Review.
Cited by
- Intramuscular injection of vectorized-scFvMC1 reduces pathological tau in two different tau transgenic models.
Vitale F, Ortolan J, Volpe BT, Marambaud P, Giliberto L, d'Abramo C. Vitale F, et al. Acta Neuropathol Commun. 2020 Aug 6;8(1):126. doi: 10.1186/s40478-020-01003-7. Acta Neuropathol Commun. 2020. PMID: 32762731 Free PMC article. - Specific serum antibody binding to phosphorylated and non-phosphorylated tau in non-cognitively impaired, mildly cognitively impaired, and Alzheimer's disease subjects: an exploratory study.
Klaver AC, Coffey MP, Bennett DA, Loeffler DA. Klaver AC, et al. Transl Neurodegener. 2017 Nov 24;6:32. doi: 10.1186/s40035-017-0100-x. eCollection 2017. Transl Neurodegener. 2017. PMID: 29204273 Free PMC article. - Tau Immunotherapies for Alzheimer's Disease and Related Tauopathies: Progress and Potential Pitfalls.
Sigurdsson EM. Sigurdsson EM. J Alzheimers Dis. 2018;64(s1):S555-S565. doi: 10.3233/JAD-179937. J Alzheimers Dis. 2018. PMID: 29865056 Free PMC article. Review. - Crystal structure of a conformational antibody that binds tau oligomers and inhibits pathological seeding by extracts from donors with Alzheimer's disease.
Abskharon R, Seidler PM, Sawaya MR, Cascio D, Yang TP, Philipp S, Williams CK, Newell KL, Ghetti B, DeTure MA, Dickson DW, Vinters HV, Felgner PL, Nakajima R, Glabe CG, Eisenberg DS. Abskharon R, et al. J Biol Chem. 2020 Jul 31;295(31):10662-10676. doi: 10.1074/jbc.RA120.013638. Epub 2020 Jun 3. J Biol Chem. 2020. PMID: 32493775 Free PMC article. - New Features about Tau Function and Dysfunction.
Medina M, Hernández F, Avila J. Medina M, et al. Biomolecules. 2016 Apr 19;6(2):21. doi: 10.3390/biom6020021. Biomolecules. 2016. PMID: 27104579 Free PMC article. Review.
References
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–9. . - PubMed
- Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging. 1997;18(4 Suppl):S85–8. . - PubMed
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. . - PubMed
- Braak H, Del Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer's disease. Adv Anat Embryol Cell Biol. 2015;215:1–162. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous