West Nile Virus Encephalitis 16 Years Later - PubMed (original) (raw)
Review
West Nile Virus Encephalitis 16 Years Later
Bette K Kleinschmidt-DeMasters et al. Brain Pathol. 2015 Sep.
Abstract
Arboviruses (Arthropod-borne viruses) include several families of viruses (Flaviviridae, Togaviradae, Bunyaviradae, Reoviradae) that are spread by arthropod vectors, most commonly mosquitoes, ticks and sandflies. The RNA genome allows these viruses to rapidly adapt to ever-changing host and environmental conditions. Thus, these virus families are largely responsible for the recent expansion in geographic range of emerging viruses including West Nile virus (WNV), dengue virus and Chikungunya virus. This review will focus on WNV, especially as it has progressively spread westward in North America since its introduction in New York in 1999. By 2003, WNV infections in humans had reached almost all lower 48 contiguous United States (US) and since that time, fluctuations in outbreaks have occurred. Cases decreased between 2008 and 2011, followed by a dramatic flair in 2012, with the epicenter in the Dallas-Fort Worth region of Texas. The 2012 outbreak was associated with an increase in reported neuroinvasive cases. Neuroinvasive disease continues to be a problem particularly in the elderly and immunocompromised populations, although WNV infections also represented the second most frequent cause of pediatric encephalitis in these same years. Neuropathological features in cases from the 2012 epidemic highlight the extent of viral damage that can occur in the CNS.
Keywords: arbovirus; epidemic; infection; neuropathology.
© 2015 International Society of Neuropathology.
Figures
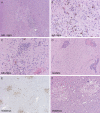
Figure 1
A. Severe zonal cohesive necrosis occupying almost the entire substantia nigra (sub. nigra), with B, profuse microglial influx, and C, foci of necrosis; all cases were severely affected at this site; three different cases illustrated. Caudate (D) was often involved, with diffuse microgliosis and, in this case, profuse perivascular lymphocytic cuffing. Thalamus was usually severely affected along with brainstem and multifocal disease could be highlighted at lower power by
CD68
immunohistochemistry (E); acutely ischemic foci showed rarefaction, necrotic neurons, and sometimes minimal cellular response (F).
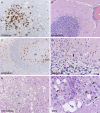
Figure 2
(A) Thalamus here shows necrotic foci with
CD68
positive macrophages. Cerebellum was often severely affected by Purkinje cell, but not granule cell, neuronal loss (B–D), microgliosis of the molecular layer (m) (B–D), neuronophagia (D, arrow). Red nucleus could manifest microglial clusters or overt pallor, vacuolization and necrosis, as seen here (E). Basis pontis was frequently severely affected and in this case, multifocal ovoid areas of necrosis contained dystrophic basophilic axonal mineralization (F, upper left); without the admixed macrophages (F, arrows) and more typical non‐mineralized foci elsewhere, this might be construed as pontine leukodystrophy.
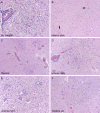
Figure 3
The same anatomical
CNS
/
PNS
sites are involved in severely immunocompromised patients as in patients with less severe comorbidities, but they are simply of larger volume and more necrotic; however, occasionally additional sites can be affected such as lateral medullary tegmentum (A), inferior olivary nucleus (B, arrows on microglial clusters), or dorsal gray matter of spinal cord (c, cc = central canal, vs = ventral sulcus). Near‐total cross‐sectional diameter of anterior horn (C,D), with neuronal loss, microglial clusters and neuronophagia (E) can be seen. The extension of inflammation in continuity from dorsal gray matter to anterior horn cell region, with involvement of
C
larke's column nucleus (F) lends histological support to theories of possible spread from
PNS
nerve roots to
CNS
.
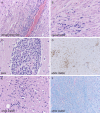
Figure 4
Dorsal nerve root of spinal cord (A) from the mid‐thoracic region shows neuritis, as was noted in the original cases of
WNVE
, but severely immunocompromised patients may also show cranial nerve neuritis (B). Extent of lymphocytic response is variable from patient to patient and sometimes even site to site, but at times can consist of very activated lymphocytes (E); however small cytologically bland lymphocytes (A,B) are the rule. Despite the very minimal involvement of cerebral cortical gray matter even in these severely affected patients, cerebral white matter can show microgliosis (D,E), the extent of which is highlighted by
CD68
immunohistochemistry on low power (D); myelin pallor, however, is usually minimal (F, Luxol fast blue‐periodic acid
S
chiff).
Similar articles
- [The occurrence of neuroinvasive symptoms caused by the West Nile virus at an emergency center].
Koch M, Török KT, Nagy F, Soós V, Pozsgai É, Lelovics Z, Nagy A, Varga C. Koch M, et al. Orv Hetil. 2019 Dec;160(51):2026-2035. doi: 10.1556/650.2019.31575. Orv Hetil. 2019. PMID: 31838862 Review. Hungarian. - Surveillance for West Nile Virus Disease - United States, 2009-2018.
McDonald E, Mathis S, Martin SW, Staples JE, Fischer M, Lindsey NP. McDonald E, et al. MMWR Surveill Summ. 2021 Mar 5;70(1):1-15. doi: 10.15585/mmwr.ss7001a1. MMWR Surveill Summ. 2021. PMID: 33661868 Free PMC article. - The evolving epidemiology of viral encephalitis.
Sejvar JJ. Sejvar JJ. Curr Opin Neurol. 2006 Aug;19(4):350-7. doi: 10.1097/01.wco.0000236613.74462.4c. Curr Opin Neurol. 2006. PMID: 16914972 Review. - West Nile virus disease and other arboviral diseases--United States, 2010.
Centers for Disease Control and Prevention (CDC). Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2011 Aug 5;60(30):1009-13. MMWR Morb Mortal Wkly Rep. 2011. PMID: 21814163 - Comparison of Characteristics of Patients with West Nile Virus or St. Louis Encephalitis Virus Neuroinvasive Disease During Concurrent Outbreaks, Maricopa County, Arizona, 2015.
Venkat H, Krow-Lucal E, Kretschmer M, Sylvester T, Levy C, Adams L, Fitzpatrick K, Laven J, Kosoy O, Sunenshine R, Smith K, Townsend J, Chevinsky J, Hennessey M, Jones J, Komatsu K, Fischer M, Hills S. Venkat H, et al. Vector Borne Zoonotic Dis. 2020 Aug;20(8):624-629. doi: 10.1089/vbz.2019.2572. Epub 2020 Apr 6. Vector Borne Zoonotic Dis. 2020. PMID: 32251616 Free PMC article.
Cited by
- Pharmacophore anchor models of flaviviral NS3 proteases lead to drug repurposing for DENV infection.
Pathak N, Lai ML, Chen WY, Hsieh BW, Yu GY, Yang JM. Pathak N, et al. BMC Bioinformatics. 2017 Dec 28;18(Suppl 16):548. doi: 10.1186/s12859-017-1957-5. BMC Bioinformatics. 2017. PMID: 29297305 Free PMC article. - Mini-Symposium: Emerging Viral Infections of the Central Nervous System.
Wiley CA. Wiley CA. Brain Pathol. 2015 Sep;25(5):598-9. doi: 10.1111/bpa.12283. Brain Pathol. 2015. PMID: 26276022 Free PMC article. No abstract available. - Forecasting the West Nile Virus in the United States: An Extensive Novel Data Streams-Based Time Series Analysis and Structural Equation Modeling of Related Digital Searching Behavior.
Watad A, Watad S, Mahroum N, Sharif K, Amital H, Bragazzi NL, Adawi M. Watad A, et al. JMIR Public Health Surveill. 2019 Feb 28;5(1):e9176. doi: 10.2196/publichealth.9176. JMIR Public Health Surveill. 2019. PMID: 30601755 Free PMC article. - Environmental and Sociological Factors Associated with the Incidence of West Nile Virus Cases in the Northern San Joaquin Valley of California, 2011-2015.
Hernandez E, Torres R, Joyce AL. Hernandez E, et al. Vector Borne Zoonotic Dis. 2019 Nov;19(11):851-858. doi: 10.1089/vbz.2019.2437. Epub 2019 Jun 18. Vector Borne Zoonotic Dis. 2019. PMID: 31211639 Free PMC article. - Nervous System Manifestations of Arboviral Infections.
Chauhan L, Matthews E, Piquet AL, Henao-Martinez A, Franco-Paredes C, Tyler KL, Beckham D, Pastula DM. Chauhan L, et al. Curr Trop Med Rep. 2022;9(4):107-118. doi: 10.1007/s40475-022-00262-9. Epub 2022 Sep 15. Curr Trop Med Rep. 2022. PMID: 36124288 Free PMC article. Review.
References
- Bains HS, Jampol LM, Caughron MC, Parnell JR (2003) Vitritis and chorioretinitis in a patient with West Nile virus infection. Arch Ophthalmol 121:205–207. - PubMed
- Biggerstaff BJ, Peterson LR (2002) Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 42:1019–1026. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources