Design considerations for nanotherapeutics in oncology - PubMed (original) (raw)
Review
Design considerations for nanotherapeutics in oncology
Triantafyllos Stylianopoulos et al. Nanomedicine. 2015 Nov.
Abstract
Nanotherapeutics have improved the quality of life of cancer patients, primarily by reducing the adverse effects of chemotherapeutic agents, but improvements in overall survival are modest. This is in large part due to the fact that the enhanced permeability and retention effect, which is the basis for the use of nanoparticles in cancer, can be also a barrier to the delivery of nanomedicines. A careful design of nanoparticle formulations can overcome barriers posed by the tumor microenvironment and result in better treatments. In this review, we first discuss strengths and limitations of clinically-approved nanoparticles. Then, we evaluate design parameters that can be modulated to optimize delivery. The benefits of active tumor targeting and drug release rate on intratumoral delivery and treatment efficacy are also discussed. Finally, we suggest specific design strategies that should optimize delivery to most solid tumors and discuss under what conditions active targeting would be beneficial.
From the clinical editor: Advances in nanotechnology have seen the introduction of new treatment modalities for cancer. The principle of action using nanocarriers for drug delivery is based mostly on the Enhanced Permeability and Retention effect. This phenomenon however, can also be a hindrance. In this article, the authors performed an in-depth review on various nanoparticle platforms in cancer therapeutics. They also suggested options to improve drug delivery, in terms of carrier design.
Keywords: Cancer therapy; Controlled drug release; EPR effect; Nanomedicine; Nanoparticle targeting.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests: R.K.J. received research grants from Dyax, Med Immune and Roche; consultaning fees from Enlight, Ophthotech, SPARC and SynDevRx; owns equity in Enlight, Ophthotech, SynDevRx and XTuit, serves on the Board of Directors of XTuit and Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. No reagents or funding from these companies was used in these studies. Therefore, there is no significant financial or other competing interest in the work.
Figures
Figure 1
Chronology of first clinical approvals of nanomedicines after the introduction of the EPR effect. Myocet is approved only in Europe and Canada, Lipusu in China, Genexol-PM in South Korea and PICN in India. The size of nanoparticles given in parenthesis is approximate. *Abraxane becomes ~10 nm in size from ~130 nm following disintegration in the blood . The size of Lipusu is not available.
Figure 2
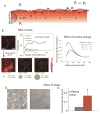
Effect of particle physicochemical properties on transvascular transport. A.) Interstitial fluid pressure, Pi, in solid tumors is elevated and is approximately equal to microvascular pressure, Pv, which eliminates transvascular pressure gradients and renders diffusion the dominant mechanism of transport. Spherical particles move with the flow, while elongated particles rotate as they move and interact with the vessel wall. Cationic particles are concentrated near the vessel wall owing to electrostatic attractions. B.) Transvascular transport of nanoparticles is size-dependent, with small nanoparticles, less than 60 nm in diameter, able to effectively extravasate (adapted with permission from ). C.) Cationic nanoparticles have superior transvascular flux in solid tumors, with q [Coulomb/m2] being the surface charge density (simulation results obtained with permission from ) and D. and E.) Rods can more effectively extravasate into the tumor compared with spherical particles of the same hydrodynamic diameter. Asterisk denotes a statistically significant difference (obtained with permission from ).
Figure 3
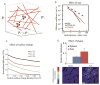
Effect of particle physicochemical properties on interstitial transport. A.) Interstitial fluid pressure is often uniformly elevated rendering diffusion the main mechanism of interstitial transport within the pores of the collagen network in the tumor interstitial space (adapted with permission from ). B.) Size-dependent interstitial diffusivity as a function of the hydrodynamic radius for diffusion in PBS, the dorsal skin and the brain (with permission from ). C.) Simulation results for the effect of electrostatic repulsion on interstitial diffusivity. Normalized diffusion is the ratio of the diffusion coefficient in the tissue over the diffusion coefficient in water and λ is the ratio of particle radius divided by the fiber radius (with permission from ). D.) Effect of particle shape on the interstitial transport of nanoparticles. Intratumor distribution refers to the area of tumor sections occupied by the particles and the distribution of the spherical and rod-like particles in a tumor section (Bottom). Asterisk denotes a statistically significant difference (with permission from ).
Figure 4
Effect of binding affinity and drug release rate on the efficacy of two-stage and multistage drug delivery systems. A.) Conventional, two-stage delivery systems are composed of the nano-carrier and the drug. The drug is released by the nano-carrier in a controlled fashion. B.) Optimization contour plot of the fraction of killed cells as a function of the binding affinity of the drug and the drug release rate. C.) Multi-stage drug delivery systems consist of an extra step in the delivery of nanomedicines to solid tumors including a primary nanoparticle, a secondary nanoparticle and the drug. D.) Optimization contour plot for a multi-stage delivery system (Adapted with permission from ).
Similar articles
- Engineered nanoparticles for drug delivery in cancer therapy.
Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Sun T, et al. Angew Chem Int Ed Engl. 2014 Nov 10;53(46):12320-64. doi: 10.1002/anie.201403036. Epub 2014 Oct 7. Angew Chem Int Ed Engl. 2014. PMID: 25294565 Review. - Improved Targeting of Cancers with Nanotherapeutics.
Foster C, Watson A, Kaplinsky J, Kamaly N. Foster C, et al. Methods Mol Biol. 2017;1530:13-37. doi: 10.1007/978-1-4939-6646-2_2. Methods Mol Biol. 2017. PMID: 28150194 - Prodrugs in combination with nanocarriers as a strategy for promoting antitumoral efficiency.
Lin MH, Hung CF, Hsu CY, Lin ZC, Fang JY. Lin MH, et al. Future Med Chem. 2019 Aug;11(16):2131-2150. doi: 10.4155/fmc-2018-0388. Future Med Chem. 2019. PMID: 31538520 Review. - Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives.
Ali ES, Sharker SM, Islam MT, Khan IN, Shaw S, Rahman MA, Uddin SJ, Shill MC, Rehman S, Das N, Ahmad S, Shilpi JA, Tripathi S, Mishra SK, Mubarak MS. Ali ES, et al. Semin Cancer Biol. 2021 Feb;69:52-68. doi: 10.1016/j.semcancer.2020.01.011. Epub 2020 Jan 31. Semin Cancer Biol. 2021. PMID: 32014609 Review. - Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance.
Chen S, Yang K, Tuguntaev RG, Mozhi A, Zhang J, Wang PC, Liang XJ. Chen S, et al. Nanomedicine. 2016 Feb;12(2):269-86. doi: 10.1016/j.nano.2015.10.020. Epub 2015 Dec 17. Nanomedicine. 2016. PMID: 26707818 Free PMC article. Review.
Cited by
- Prostate-Specific Membrane Antigen Targeted Deep Tumor Penetration of Polymer Nanocarriers.
Meher N, Ashley GW, Bidkar AP, Dhrona S, Fong C, Fontaine SD, Beckford Vera DR, Wilson DM, Seo Y, Santi DV, VanBrocklin HF, Flavell RR. Meher N, et al. ACS Appl Mater Interfaces. 2022 Nov 16;14(45):50569-50582. doi: 10.1021/acsami.2c15095. Epub 2022 Nov 1. ACS Appl Mater Interfaces. 2022. PMID: 36318757 Free PMC article. - Assessing the gene silencing potential of AuNP-based approaches on conventional 2D cell culture versus 3D tumor spheroid.
Oliveira BB, Fernandes AR, Baptista PV. Oliveira BB, et al. Front Bioeng Biotechnol. 2024 Feb 12;12:1320729. doi: 10.3389/fbioe.2024.1320729. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38410164 Free PMC article. - Normalizing tumor microenvironment with nanomedicine and metronomic therapy to improve immunotherapy.
Mpekris F, Voutouri C, Panagi M, Baish JW, Jain RK, Stylianopoulos T. Mpekris F, et al. J Control Release. 2022 May;345:190-199. doi: 10.1016/j.jconrel.2022.03.008. Epub 2022 Mar 8. J Control Release. 2022. PMID: 35271911 Free PMC article. - UV scintillating particles as radiosensitizer enhance cell killing after X-ray excitation.
Müller M, Wang Y, Squillante MR, Held KD, Anderson RR, Purschke M. Müller M, et al. Radiother Oncol. 2018 Dec;129(3):589-594. doi: 10.1016/j.radonc.2018.06.016. Epub 2018 Jun 29. Radiother Oncol. 2018. PMID: 30539764 Free PMC article. - Structure of the Anti-C60 Fullerene Antibody Fab Fragment: Structural Determinants of Fullerene Binding.
Osipov EM, Hendrickson OD, Tikhonova TV, Zherdev AV, Solopova ON, Sveshnikov PG, Dzantiev BB, Popov VO. Osipov EM, et al. Acta Naturae. 2019 Jan-Mar;11(1):58-65. Acta Naturae. 2019. PMID: 31024749 Free PMC article.
References
- Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. - PubMed
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- R01-CA098706/CA/NCI NIH HHS/United States
- R01 CA098706/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources