Plasma metabolomic profiles enhance precision medicine for volunteers of normal health - PubMed (original) (raw)
. 2015 Sep 1;112(35):E4901-10.
doi: 10.1073/pnas.1508425112. Epub 2015 Aug 17.
Michael V Milburn 2, John A Ryals 2, Shaun C Lonergan 2, Matthew W Mitchell 2, Jacob E Wulff 2, Danny C Alexander 2, Anne M Evans 2, Brandi Bridgewater 2, Luke Miller 2, Manuel L Gonzalez-Garay 3, C Thomas Caskey 4
Affiliations
- PMID: 26283345
- PMCID: PMC4568216
- DOI: 10.1073/pnas.1508425112
Plasma metabolomic profiles enhance precision medicine for volunteers of normal health
Lining Guo et al. Proc Natl Acad Sci U S A. 2015.
Abstract
Precision medicine, taking account of human individuality in genes, environment, and lifestyle for early disease diagnosis and individualized therapy, has shown great promise to transform medical care. Nontargeted metabolomics, with the ability to detect broad classes of biochemicals, can provide a comprehensive functional phenotype integrating clinical phenotypes with genetic and nongenetic factors. To test the application of metabolomics in individual diagnosis, we conducted a metabolomics analysis on plasma samples collected from 80 volunteers of normal health with complete medical records and three-generation pedigrees. Using a broad-spectrum metabolomics platform consisting of liquid chromatography and GC coupled with MS, we profiled nearly 600 metabolites covering 72 biochemical pathways in all major branches of biosynthesis, catabolism, gut microbiome activities, and xenobiotics. Statistical analysis revealed a considerable range of variation and potential metabolic abnormalities across the individuals in this cohort. Examination of the convergence of metabolomics profiles with whole-exon sequences (WESs) provided an effective approach to assess and interpret clinical significance of genetic mutations, as shown in a number of cases, including fructose intolerance, xanthinuria, and carnitine deficiency. Metabolic abnormalities consistent with early indications of diabetes, liver dysfunction, and disruption of gut microbiome homeostasis were identified in several volunteers. Additionally, diverse metabolic responses to medications among the volunteers may assist to identify therapeutic effects and sensitivity to toxicity. The results of this study demonstrate that metabolomics could be an effective approach to complement next generation sequencing (NGS) for disease risk analysis, disease monitoring, and drug management in our goal toward precision care.
Keywords: disease assessment; functional phenotyping; gene penetrance; metabolomics; whole-exome sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Classification of the plasma metabolites detected in this study. (A) Total number of metabolites based on their biochemical classes. (B) Metabolites modulated by gut bacteria activities. Nonitalicized metabolites are exclusively or mainly contributed by bacteria metabolism, and italicized metabolites are jointly contributed by both mammalian cells and bacteria.
Fig. 2.
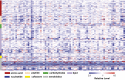
Metabolomics diversity of the cohort illustrated by the heat map of the metabolomic profiles of the volunteers. Red and blue indicate high and low levels, respectively, relative to the median value for all samples (median = 1.0). The workflow to generate new candidate genes from metabolomics data is shown in Fig. 3. The vCard files are annotated using spnEff and ANNOVAR. Nonsynonymous coding variants are identified. A list of genes corresponding to an abnormal metabolic pathway is generated, and nonsynonymous coding variants are isolated. Variants are filtered using frequency and functional effect filters. Details of the process and methods are provided in Materials and Methods.
Fig. 3.
Work flow for searching WES data and metabolomics convergence. MAF, minor allele frequency.
Fig. 4.
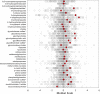
Assessment of the metabolic perturbations defined biochemical pathways. (A) Purine degradation pathway and dot plots showing data distribution in the cohort for xanthine, urate, and hypoxanthine. The red dots show the metabolite level for volunteer 3923. The open dots show the data distribution for the rest of the cohort (n = 80). (B) Sorbitol degradation pathway and dot plots showing data distribution in the cohort for fructose and sorbitol. The red dots show the metabolite level for volunteer 3905. The open dots show the data distribution for the rest of the cohort (n = 80). The box represents the middle 50% of the distribution, and left and right ‘‘whiskers’’ represent the entire spread of the data. The vertical line refers to the median, and the plus symbol refers to the mean. The first and second numbers within the parentheses are the z-score and P value, respectively.
Fig. 5.
Data distribution for long-chain fatty acid carnitines and 3-methylhistidine in the cohort (n = 80). The red dots show the metabolite level for volunteer 3890. The open dots show the data distribution for the rest of the cohort. An explanation of the plots is provided in the legend for Fig. 4. The first and second numbers within the parentheses are the z-score and P value, respectively.
Fig. 6.
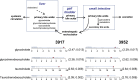
Bile acid circulation and dot plots showing data distribution in the cohort (n = 80) for the four primary bile acids. The red dots show the metabolite level for either volunteer 3917 or volunteer 3952. The open dots show the data distribution for the rest of the cohort. The box represents the middle 50% of the distribution, and left and right ‘‘whiskers’’ represent the entire spread of the data. The vertical line refers to the median, and the plus refers to the mean. An explanation of the plots is provided in the legend for Fig. 4. The first and second numbers within the parentheses are the z-score and P value, respectively.
Fig. 7.
Condensed metabolic schemes for energy metabolism and dot plots showing data distribution in the cohort (n = 80) for key metabolites with a known association with diabetes. The red dots show the metabolite level for the specific volunteers as labeled next to the plots. The open dots show the data distribution for the rest of the cohort. An explanation of the plots is provided in the legend for Fig. 4. The first and second numbers within the parentheses are the z-score and P value, respectively.
Fig. 8.
Distribution of key metabolites with known association with diabetes in the cohort (n = 80). The red dots show the metabolite level for either volunteer 3891 or volunteer 3837. The open dots show the data distribution for the rest of the cohort. An explanation of the plots is provided in the legend for Fig. 4. The first and second numbers within the parenthesis are the z-score and P value, respectively.
Fig. 9.
Dot plots showing data distribution in the cohort (n = 80) for the acetaminophen metabolites, four primary bile acids, and GSH. The red dots show the metabolite level for either volunteer 3976 or volunteer 3958. The open dots show the data distribution for the rest of the cohort. An explanation of the plots is provided in the legend for Fig. 3. The first and second numbers within the parenthesis are the z-score and P value, respectively.
Fig. 10.
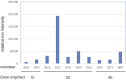
Relative serum level of atorvastatin and daily dose among the 10 volunteers taking Lipitor.
Fig. S1.
Data distribution for gut bacteria-derived metabolites in the cohort. The red dots show the metabolite level for volunteer 3930. The open dots show the data distribution for the rest of the cohort.
Similar articles
- A Simultaneous Metabolic Profiling and Quantitative Multimetabolite Metabolomic Method for Human Plasma Using Gas-Chromatography Tandem Mass Spectrometry.
Savolainen OI, Sandberg AS, Ross AB. Savolainen OI, et al. J Proteome Res. 2016 Jan 4;15(1):259-65. doi: 10.1021/acs.jproteome.5b00790. Epub 2015 Nov 30. J Proteome Res. 2016. PMID: 26615962 - Postprandial metabolomics: A pilot mass spectrometry and NMR study of the human plasma metabolome in response to a challenge meal.
Karimpour M, Surowiec I, Wu J, Gouveia-Figueira S, Pinto R, Trygg J, Zivkovic AM, Nording ML. Karimpour M, et al. Anal Chim Acta. 2016 Feb 18;908:121-31. doi: 10.1016/j.aca.2015.12.009. Epub 2015 Dec 17. Anal Chim Acta. 2016. PMID: 26826694 - Metabolomics enables precision medicine: "A White Paper, Community Perspective".
Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, Brennan L, Wishart DS, Oresic M, Hankemeier T, Broadhurst DI, Lane AN, Suhre K, Kastenmüller G, Sumner SJ, Thiele I, Fiehn O, Kaddurah-Daouk R; for “Precision Medicine and Pharmacometabolomics Task Group”-Metabolomics Society Initiative. Beger RD, et al. Metabolomics. 2016;12(10):149. doi: 10.1007/s11306-016-1094-6. Epub 2016 Sep 2. Metabolomics. 2016. PMID: 27642271 Free PMC article. - Untargeted Plasma Metabolomics and Gut Microbiome Profiling Provide Novel Insights into the Regulation of Platelet Reactivity in Healthy Individuals.
Vadaq N, Schirmer M, Tunjungputri RN, Vlamakis H, Chiriac C, Ardiansyah E, Gasem MH, Joosten LAB, de Groot PG, Xavier RJ, Netea MG, van der Ven AJ, de Mast Q. Vadaq N, et al. Thromb Haemost. 2022 Apr;122(4):529-539. doi: 10.1055/a-1541-3706. Epub 2021 Aug 13. Thromb Haemost. 2022. PMID: 34192775 Review. - [Blood metabolome analysis for creating a digital image of a healthy person].
Trifonova OP, Balashova EE, Maslov DL, Grigoriev AI, Lisitsa AV, Ponomarenko EA, Archakov AI. Trifonova OP, et al. Biomed Khim. 2020 May;66(3):216-223. doi: 10.18097/PBMC20206603216. Biomed Khim. 2020. PMID: 32588827 Review. Russian.
Cited by
- Metabolic Profiling of Aromatic Compounds.
Pautova AK. Pautova AK. Metabolites. 2024 Feb 5;14(2):107. doi: 10.3390/metabo14020107. Metabolites. 2024. PMID: 38392999 Free PMC article. - Early-Life Iron Deficiency and Its Natural Resolution Are Associated with Altered Serum Metabolomic Profiles in Infant Rhesus Monkeys.
Sandri BJ, Lubach GR, Lock EF, Georgieff MK, Kling PJ, Coe CL, Rao RB. Sandri BJ, et al. J Nutr. 2020 Apr 1;150(4):685-693. doi: 10.1093/jn/nxz274. J Nutr. 2020. PMID: 31722400 Free PMC article. - Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: mini review.
Yu L, Li K, Zhang X. Yu L, et al. Oncotarget. 2017 Nov 11;8(70):115774-115786. doi: 10.18632/oncotarget.22404. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383200 Free PMC article. Review. - Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: towards personalized drug therapy.
Kantae V, Krekels EHJ, Esdonk MJV, Lindenburg P, Harms AC, Knibbe CAJ, Van der Graaf PH, Hankemeier T. Kantae V, et al. Metabolomics. 2017;13(1):9. doi: 10.1007/s11306-016-1143-1. Epub 2016 Dec 19. Metabolomics. 2017. PMID: 28058041 Free PMC article. - Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites.
Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, Brewerton S, Turpaz Y, Perkins BA, Evans AM, Miller LA, Guo L, Caskey CT, Schork NJ, Garner C, Spector TD, Venter JC, Telenti A. Long T, et al. Nat Genet. 2017 Apr;49(4):568-578. doi: 10.1038/ng.3809. Epub 2017 Mar 6. Nat Genet. 2017. PMID: 28263315
References
- Maxmen A. Exome sequencing deciphers rare diseases. Cell. 2011;144(5):635–637. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous