The Beneficial Effect of Equisetum giganteum L. against Candida Biofilm Formation: New Approaches to Denture Stomatitis - PubMed (original) (raw)
The Beneficial Effect of Equisetum giganteum L. against Candida Biofilm Formation: New Approaches to Denture Stomatitis
Rafaela A S Alavarce et al. Evid Based Complement Alternat Med. 2015.
Abstract
Equisetum giganteum L. (E. giganteum), Equisetaceae, commonly called "giant horsetail," is an endemic plant of Central and South America and is used in traditional medicine as diuretic and hemostatic in urinary disorders and in inflammatory conditions among other applications. The chemical composition of the extract EtOH 70% of E. giganteum has shown a clear presence of phenolic compounds derived from caffeic and ferulic acids and flavonoid heterosides derived from quercitin and kaempferol, in addition to styrylpyrones. E. giganteum, mainly at the highest concentrations, showed antimicrobial activity against the relevant microorganisms tested: Escherichia coli, Staphylococcus aureus, and Candida albicans. It also demonstrated antiadherent activity on C. albicans biofilms in an experimental model that is similar to dentures. Moreover, all concentrations tested showed anti-inflammatory activity. The extract did not show cytotoxicity in contact with human cells. These properties might qualify E. giganteum extract to be a promising alternative for the topic treatment and prevention of oral candidiasis and denture stomatitis.
Figures
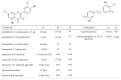
Figure 1
Structure of the flavonoids and styrylpyrones identified by UHPLC-PAD-ESI-MS_n_ in the hydroethanolic extract of E. giganteum.
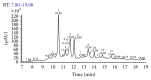
Figure 2
UHPLC-PAD analytical chromatogram of the 70% EtOH extract of the aerial parts of E. giganteum at λ = 254 nm. Mobile phase was water ultrapure (eluent A) and methanol (eluent B), both containing 0.1% of formic acid. The ratio was 5–100 of A in B in 20 min. Injection volume: 10.0 _μ_L; Thermo Scientific column (50 × 2.1 mm, 1.9 _μ_m); column temperature: 25°C; flow ratio: 0.4 mL·min−1.
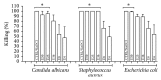
Figure 3
Median ± S.D. of percentage of killing against E. coli O:124, S. aureus ATCC 6538, and C. albicans SC 5314, after 24 hours in contact with extract at different concentrations (mg/mL): 50 (E50), 25 (E25), 16 (E16), 8 (E8), and 4 (E4). The respective controls were incubated with 1% sodium hypochlorite (CTRL/NaOCl) or with culture medium (CTRL/Medium), which showed no killing of microorganisms (0%). ∗ p < 0.05 represents a significant change compared with the CTRL/Medium. Five independent experiments were performed in triplicate (n = 15).
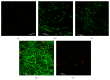
Figure 4
Confocal microscopy images of Candida albicans biofilms after each treatment with extract. (a) E50; (b) E25; (c) E16; (d) CTRL/PBS; and (e) CTRL/NaOCl. Six independent experiments were performed in triplicate.
Figure 5
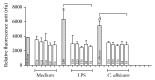
Production of ROS by human monocyte cells cultured and treated with LPS and C. albicans in the presence or absence of extracts as described in Materials and Methods. Results are expressed as mean ± S.D. of at least four experiments. Symbols indicate significant difference (p < 0.05): _∗_in comparison with LPS-treated cells and δ compared with _C. albicans_-treated cells, in the presence of extract in all concentrations tested.
Figure 6
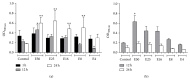
(a) Effects of E. giganteum on cell viability of human monocytes. The viability of cells under treatments with different concentrations of E. giganteum was investigated by MTT assays. Data shown are the mean ± S.D. of at least four independent experiments. Asterisks indicate significant difference compared with the control (p < 0.05). (b) The effects of E. giganteum on cell viability of HEPCs. The viability of cells under treatments with different concentrations of E. giganteum was investigated by MTT assays. Data shown are the mean ± S.D. of at least three independent experiments; ∗ p < 0.05 represents a significant change compared with the control.
Similar articles
- Equisetum giganteum influences the ability of Candida albicans in forming biofilms over the denture acrylic resin surface.
da Silva RA, Bernardo LP, Moreno JML, Lara VS, Porto VC. da Silva RA, et al. Pharm Biol. 2017 Dec;55(1):1698-1702. doi: 10.1080/13880209.2017.1321024. Pharm Biol. 2017. PMID: 28454505 Free PMC article. - Identification of phenolic compounds in Equisetum giganteum by LC-ESI-MS/MS and a new approach to total flavonoid quantification.
Francescato LN, Debenedetti SL, Schwanz TG, Bassani VL, Henriques AT. Francescato LN, et al. Talanta. 2013 Feb 15;105:192-203. doi: 10.1016/j.talanta.2012.11.072. Epub 2012 Dec 2. Talanta. 2013. PMID: 23598008 - Antimicrobial activity of denture adhesive associated with Equisetum giganteum- and Punica granatum-enriched fractions against Candida albicans biofilms on acrylic resin surfaces.
Almeida NLM, Saldanha LL, da Silva RA, Pinke KH, da Costa EF, Porto VC, Dokkedal AL, Lara VS. Almeida NLM, et al. Biofouling. 2018 Jan;34(1):62-73. doi: 10.1080/08927014.2017.1407408. Epub 2017 Dec 18. Biofouling. 2018. PMID: 29250982 - Candida albicans importance to denture wearers. A literature review.
Gleiznys A, Zdanavičienė E, Žilinskas J. Gleiznys A, et al. Stomatologija. 2015;17(2):54-66. Stomatologija. 2015. PMID: 26879270 Review. - Factors involved in microbial colonization of oral prostheses.
von Fraunhofer JA, Loewy ZG. von Fraunhofer JA, et al. Gen Dent. 2009 Mar-Apr;57(2):136-43; quiz 144-5. Gen Dent. 2009. PMID: 19552363 Review.
Cited by
- Natural Compounds for Preventing Ear, Nose, and Throat-Related Oral Infections.
Niculescu AG, Grumezescu AM. Niculescu AG, et al. Plants (Basel). 2021 Sep 6;10(9):1847. doi: 10.3390/plants10091847. Plants (Basel). 2021. PMID: 34579380 Free PMC article. Review. - Ethnopharmacological Potential of Phytochemicals and Phytogenic Products against Human RNA Viral Diseases as Preventive Therapeutics.
Paul A, Chakraborty N, Sarkar A, Acharya K, Ranjan A, Chauhan A, Srivastava S, Singh AK, Rai AK, Mubeen I, Prasad R. Paul A, et al. Biomed Res Int. 2023 Feb 20;2023:1977602. doi: 10.1155/2023/1977602. eCollection 2023. Biomed Res Int. 2023. PMID: 36860811 Free PMC article. Review. - Heat-Polymerized Resin Containing Dimethylaminododecyl Methacrylate Inhibits Candida albicans Biofilm.
Chen H, Han Q, Zhou X, Zhang K, Wang S, Xu HHK, Weir MD, Feng M, Li M, Peng X, Ren B, Cheng L. Chen H, et al. Materials (Basel). 2017 Apr 20;10(4):431. doi: 10.3390/ma10040431. Materials (Basel). 2017. PMID: 28772791 Free PMC article. - Equisetum arvense L. Extract Induces Antibacterial Activity and Modulates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress.
Pallag A, Filip GA, Olteanu D, Clichici S, Baldea I, Jurca T, Micle O, Vicaş L, Marian E, Soriţău O, Cenariu M, Mureşan M. Pallag A, et al. Oxid Med Cell Longev. 2018 Feb 14;2018:3060525. doi: 10.1155/2018/3060525. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29636839 Free PMC article. - The impact of flavonoids-rich Ziziphus jujuba Mill. Extract on Staphylococcus aureus biofilm formation.
Miao W, Sheng L, Yang T, Wu G, Zhang M, Sun J, Ainiwaer A. Miao W, et al. BMC Complement Med Ther. 2020 Jun 17;20(1):187. doi: 10.1186/s12906-020-2833-9. BMC Complement Med Ther. 2020. PMID: 32552790 Free PMC article.
References
- Lombardi T., Budtz-Jörgensen E. Treatment of denture-induced stomatitis: a review. The European Journal of Prosthodontics and Restorative Dentistry. 1993;2(supplement 1):17–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources