Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders - PubMed (original) (raw)
Review
Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders
Sacha B Nelson et al. Neuron. 2015.
Abstract
Autism spectrum disorders (ASDs) and related neurological disorders are associated with mutations in many genes affecting the ratio between neuronal excitation and inhibition. However, understanding the impact of these mutations on network activity is complicated by the plasticity of these networks, making it difficult in many cases to separate initial deficits from homeostatic compensation. Here we explore the contrasting evidence for primary defects in inhibition or excitation in ASDs and attempt to integrate the findings in terms of the brain's ability to maintain functional homeostasis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1. Summary of brain activity mapping in Mecp2 mutant mice based on Fos expression
Low Fos expression in the motor cortex (Mctx) and adjacent regions indicates decreased activity while there is higher Fos expression in the nucleus of the solitary tract (nTS) and nearby areas. Differences in Fos expression in the Mecp2 null brain compared to wild-type are color coded as follows: Red, Null < Wt; Green, Null > Wt. Reproduced with permission from (Kron et al., 2012).
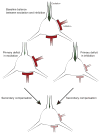
Figure 2. Homeostatic compensation regulates excitation/inhibition ratio in cortical networks
Proper neural network function relies on the balance between excitatory (green) and inhibitory (red) input. Primary defects in excitation or inhibition can be corrected via secondary compensatory mechanisms to restore balance and maintain network function. When a cell receives reduced excitation, secondary mechanisms down regulate the amount of inhibitory input onto this cell. Similarly, the excitatory input is decreased in response to a deficit in inhibition. Hence changes in both classes of synapses can appear similar following disease mechanisms that initially affect only one or the other.
Figure 3. Faithful signal propagation in multilayered cortical networks may require higher order layers to compensate for altered activity in lower layers
Cortical networks can be schematized as interconnected layers of neurons. Activity in the “input layer” of primary sensory cortices is driven by sensory inputs, while activity in higher order association and limbic regions depends to a greater degree on activity in preceding layers. (A) During normal development excitation and inhibition are balanced to preserve appropriate activity levels across synaptically connected brain regions with the activity of the cells in each layer adjusted to the amount of input this layer receives. (B) If the balance is perturbed so that, for example, input layers have reduced activity (indicated by normal red inhibitory but reduced green excitatory activity), homeostatic mechanisms compensate for the defect and upregulate the excitability of circuits in higher order Association and Limbic regions (indicated by a darker shade of green and lighter shade of red in some neurons) in an attempt to maintain normal levels of propagating activity. However, if not perfectly balanced, this can lead to overactivity in higher order regions coexisting with reduced activity in lower order regions. Networks in higher order regions with enhanced excitation and reduced inhibition may be brittle and prone to develop epileptiform activity.
Similar articles
- All for One But Not One for All: Excitatory Synaptic Scaling and Intrinsic Excitability Are Coregulated by CaMKIV, Whereas Inhibitory Synaptic Scaling Is Under Independent Control.
Joseph A, Turrigiano GG. Joseph A, et al. J Neurosci. 2017 Jul 12;37(28):6778-6785. doi: 10.1523/JNEUROSCI.0618-17.2017. Epub 2017 Jun 7. J Neurosci. 2017. PMID: 28592691 Free PMC article. - Mechanisms of GABAergic homeostatic plasticity.
Wenner P. Wenner P. Neural Plast. 2011;2011:489470. doi: 10.1155/2011/489470. Epub 2011 Aug 17. Neural Plast. 2011. PMID: 21876819 Free PMC article. Review. - Modeling of substance P and 5-HT induced synaptic plasticity in the lamprey spinal CPG: consequences for network pattern generation.
Kozlov A, Kotaleski JH, Aurell E, Grillner S, Lansner A. Kozlov A, et al. J Comput Neurosci. 2001 Sep-Oct;11(2):183-200. doi: 10.1023/a:1012806018730. J Comput Neurosci. 2001. PMID: 11717534 - The missing piece in the 'use it or lose it' puzzle: is inhibition regulated by activity or does it act on its own accord?
Sun QQ. Sun QQ. Rev Neurosci. 2007;18(3-4):295-310. doi: 10.1515/revneuro.2007.18.3-4.295. Rev Neurosci. 2007. PMID: 18019611 Free PMC article. Review. - Alterations of GABAergic signaling in autism spectrum disorders.
Pizzarelli R, Cherubini E. Pizzarelli R, et al. Neural Plast. 2011;2011:297153. doi: 10.1155/2011/297153. Epub 2011 Jun 23. Neural Plast. 2011. PMID: 21766041 Free PMC article. Review.
Cited by
- Editorial: Homeostatic Synaptic Plasticity: From Synaptic Circuit Assembly to Neurological Disorders.
Letellier M, Cingolani LA. Letellier M, et al. Front Cell Neurosci. 2021 May 14;15:695313. doi: 10.3389/fncel.2021.695313. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34054435 Free PMC article. No abstract available. - Impaired synaptic incorporation of AMPA receptors in a mouse model of fragile X syndrome.
Chojnacka M, Beroun A, Magnowska M, Stawikowska A, Cysewski D, Milek J, Dziembowska M, Kuzniewska B. Chojnacka M, et al. Front Mol Neurosci. 2023 Nov 9;16:1258615. doi: 10.3389/fnmol.2023.1258615. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38025260 Free PMC article. - Fxr1 regulates sleep and synaptic homeostasis.
Khlghatyan J, Evstratova A, Bozoyan L, Chamberland S, Chatterjee D, Marakhovskaia A, Soares Silva T, Toth K, Mongrain V, Beaulieu JM. Khlghatyan J, et al. EMBO J. 2020 Nov 2;39(21):e103864. doi: 10.15252/embj.2019103864. Epub 2020 Sep 7. EMBO J. 2020. PMID: 32893934 Free PMC article. - Trans-Synaptic Signaling through the Glutamate Receptor Delta-1 Mediates Inhibitory Synapse Formation in Cortical Pyramidal Neurons.
Fossati M, Assendorp N, Gemin O, Colasse S, Dingli F, Arras G, Loew D, Charrier C. Fossati M, et al. Neuron. 2019 Dec 18;104(6):1081-1094.e7. doi: 10.1016/j.neuron.2019.09.027. Epub 2019 Nov 5. Neuron. 2019. PMID: 31704028 Free PMC article. - A transcriptional constraint mechanism limits the homeostatic response to activity deprivation in mammalian neocortex.
Valakh V, Wise D, Zhu XA, Sha M, Fok J, Van Hooser SD, Schectman R, Cepeda I, Kirk R, O'Toole SM, Nelson SB. Valakh V, et al. Elife. 2023 Feb 7;12:e74899. doi: 10.7554/eLife.74899. Elife. 2023. PMID: 36749029 Free PMC article.
References
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. - PubMed
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. PNAS. 2009;106:4507–4512. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources