Role of dual specificity tyrosine-phosphorylation-regulated kinase 1B (Dyrk1B) in S-phase entry of HPV E7 expressing cells from quiescence - PubMed (original) (raw)
Role of dual specificity tyrosine-phosphorylation-regulated kinase 1B (Dyrk1B) in S-phase entry of HPV E7 expressing cells from quiescence
Na Zhou et al. Oncotarget. 2015.
Abstract
The high-risk human papillomavirus (HPV) is the causative agent for cervical cancer. The HPV E7 oncogene promotes S-phase entry from quiescent state in the presence of elevated cell cycle inhibitor p27Kip1, a function that may contribute to carcinogenesis. However, the mechanism by which HPV E7 induces quiescent cells to entry into S-phase is not fully understood. Interestingly, we found that Dyrk1B, a dual-specificity kinase and negative regulator of cell proliferation in quiescent cells, was upregulated in E7 expressing cells. Surprisingly and in contrast to what was previously reported, Dyrk1B played a positive role in S-phase entry of quiescent HPV E7 expressing cells. Mechanistically, Dyrk1B contributed to p27 phosphorylation (at serine 10 and threonine 198), which was important for the proliferation of HPV E7 expressing cells. Moreover, Dyrk1B up-regulated HPV E7. Taken together, our studies uncovered a novel function of Dyrk1B in high-risk HPV E7-mediated cell proliferation. Dyrk1B may serve as a target for therapy in HPV-associated cancers.
Keywords: Dyrk1B; E7; HPV; S-phase; p27; quiescence.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Figures
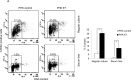
Figure 1. HPV E7 expressing cells entry into S phase under serum starvation and elevated levels of p27
Cells expressing E7 and control were cultured in the presence or absence of serum for 2 days. The cells were labeled with BrdU for 2 h before collecting and stained with 7-AAD and anti-BrdU antibody, analyzed by flow cytometry. BrdU-positive cells are gated and their percentages are indicated. A. PHKs. B. RPE1 cells. Lower panels, quantification of BrdU staining. Error bars reflect the standard deviations of the mean. C. and D. The steady-state levels of p27 in PHKs C. and RPE1 cells D. expressing E7 or control were examined by Western blot. E. RPE1 cells were plated at high density and the steady-state levels of p27 were examined. RD, regular cell density; HD, high cell density. β-tubulin was used as a loading control. A representative experiment of three is shown. Right panels, quantification p27 protein levels. *, p < 0.05; **, p < 0.01.
Figure 1. HPV E7 expressing cells entry into S phase under serum starvation and elevated levels of p27
Cells expressing E7 and control were cultured in the presence or absence of serum for 2 days. The cells were labeled with BrdU for 2 h before collecting and stained with 7-AAD and anti-BrdU antibody, analyzed by flow cytometry. BrdU-positive cells are gated and their percentages are indicated. A. PHKs. B. RPE1 cells. Lower panels, quantification of BrdU staining. Error bars reflect the standard deviations of the mean. C. and D. The steady-state levels of p27 in PHKs C. and RPE1 cells D. expressing E7 or control were examined by Western blot. E. RPE1 cells were plated at high density and the steady-state levels of p27 were examined. RD, regular cell density; HD, high cell density. β-tubulin was used as a loading control. A representative experiment of three is shown. Right panels, quantification p27 protein levels. *, p < 0.05; **, p < 0.01.
Figure 1. HPV E7 expressing cells entry into S phase under serum starvation and elevated levels of p27
Cells expressing E7 and control were cultured in the presence or absence of serum for 2 days. The cells were labeled with BrdU for 2 h before collecting and stained with 7-AAD and anti-BrdU antibody, analyzed by flow cytometry. BrdU-positive cells are gated and their percentages are indicated. A. PHKs. B. RPE1 cells. Lower panels, quantification of BrdU staining. Error bars reflect the standard deviations of the mean. C. and D. The steady-state levels of p27 in PHKs C. and RPE1 cells D. expressing E7 or control were examined by Western blot. E. RPE1 cells were plated at high density and the steady-state levels of p27 were examined. RD, regular cell density; HD, high cell density. β-tubulin was used as a loading control. A representative experiment of three is shown. Right panels, quantification p27 protein levels. *, p < 0.05; **, p < 0.01.
Figure 1. HPV E7 expressing cells entry into S phase under serum starvation and elevated levels of p27
Cells expressing E7 and control were cultured in the presence or absence of serum for 2 days. The cells were labeled with BrdU for 2 h before collecting and stained with 7-AAD and anti-BrdU antibody, analyzed by flow cytometry. BrdU-positive cells are gated and their percentages are indicated. A. PHKs. B. RPE1 cells. Lower panels, quantification of BrdU staining. Error bars reflect the standard deviations of the mean. C. and D. The steady-state levels of p27 in PHKs C. and RPE1 cells D. expressing E7 or control were examined by Western blot. E. RPE1 cells were plated at high density and the steady-state levels of p27 were examined. RD, regular cell density; HD, high cell density. β-tubulin was used as a loading control. A representative experiment of three is shown. Right panels, quantification p27 protein levels. *, p < 0.05; **, p < 0.01.
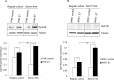
Figure 2. Dyrk1B expression and localization in HPV E7 expressing cells
The steady-state levels of Dyrk1B in PHKs A. and RPE1 cells B. expressing E7 or control were examined by Western blot. β-tubulin was used as a loading control. Lower panels, quantification of Dyrk1B protein levels. C. Dyrk1B mRNA levels in RPE1 cells expressing E7 or control were examined by real-time PCR. Expression levels were assessed in triplicate and normalized to GAPDH levels. Results from three independent experiments were summarized. Cytoplasmic and nuclear fractions were prepared from PHKs D. and RPE1 cells E. expressing HPV E7 or control and immune-blotted with antibodies specific for Dyrk1B, β-tubulin (cytoplasmic protein marker) or SP1 (nuclear marker). Equal amount of cytoplasmic proteins and nuclear proteins were loaded. C: cytoplasm; N: nucleus. Data from one representative experiment of four are shown. *, p < 0.05; **, p < 0.01.
Figure 2. Dyrk1B expression and localization in HPV E7 expressing cells
The steady-state levels of Dyrk1B in PHKs A. and RPE1 cells B. expressing E7 or control were examined by Western blot. β-tubulin was used as a loading control. Lower panels, quantification of Dyrk1B protein levels. C. Dyrk1B mRNA levels in RPE1 cells expressing E7 or control were examined by real-time PCR. Expression levels were assessed in triplicate and normalized to GAPDH levels. Results from three independent experiments were summarized. Cytoplasmic and nuclear fractions were prepared from PHKs D. and RPE1 cells E. expressing HPV E7 or control and immune-blotted with antibodies specific for Dyrk1B, β-tubulin (cytoplasmic protein marker) or SP1 (nuclear marker). Equal amount of cytoplasmic proteins and nuclear proteins were loaded. C: cytoplasm; N: nucleus. Data from one representative experiment of four are shown. *, p < 0.05; **, p < 0.01.
Figure 3. Dyrk1B promotes S phase entry in E7 expressing quiescent cells
A. RPE1-E7 cells were transfected with siRNA targeting Dyrk1B and cultured in either regular medium or serum free medium. Forty-eight hours after transfection, cells in one set of dishes were harvested, lysed and Dyrk1B protein levels were analyzed by immunoblot. β-tubulin was used as a loading control. Cells in another set of dishes were labeled with BrdU for 2 hours before collecting. Cells were then stained with anti-BrdU antibody, counterstained with 7-AAD, and analyzed by flow cytometry and quantified (Lower left panel). The percentage of BrdU-positive cells was indicated. NC, non-silencing siRNA. B. RPE1-E7 and control cells were transfected with plasmids encoding HA-Dyrk1B fusion or control vector under either serum starvation B. or regular culture condition C., and either were subjected to immunoblot analysis with anti-HA antibody, or labeled with BrdU and analyzed by flow cytometry and quantified (Lower left panel). Data from a representative of at least three experiments are shown.
Figure 3. Dyrk1B promotes S phase entry in E7 expressing quiescent cells
A. RPE1-E7 cells were transfected with siRNA targeting Dyrk1B and cultured in either regular medium or serum free medium. Forty-eight hours after transfection, cells in one set of dishes were harvested, lysed and Dyrk1B protein levels were analyzed by immunoblot. β-tubulin was used as a loading control. Cells in another set of dishes were labeled with BrdU for 2 hours before collecting. Cells were then stained with anti-BrdU antibody, counterstained with 7-AAD, and analyzed by flow cytometry and quantified (Lower left panel). The percentage of BrdU-positive cells was indicated. NC, non-silencing siRNA. B. RPE1-E7 and control cells were transfected with plasmids encoding HA-Dyrk1B fusion or control vector under either serum starvation B. or regular culture condition C., and either were subjected to immunoblot analysis with anti-HA antibody, or labeled with BrdU and analyzed by flow cytometry and quantified (Lower left panel). Data from a representative of at least three experiments are shown.
Figure 3. Dyrk1B promotes S phase entry in E7 expressing quiescent cells
A. RPE1-E7 cells were transfected with siRNA targeting Dyrk1B and cultured in either regular medium or serum free medium. Forty-eight hours after transfection, cells in one set of dishes were harvested, lysed and Dyrk1B protein levels were analyzed by immunoblot. β-tubulin was used as a loading control. Cells in another set of dishes were labeled with BrdU for 2 hours before collecting. Cells were then stained with anti-BrdU antibody, counterstained with 7-AAD, and analyzed by flow cytometry and quantified (Lower left panel). The percentage of BrdU-positive cells was indicated. NC, non-silencing siRNA. B. RPE1-E7 and control cells were transfected with plasmids encoding HA-Dyrk1B fusion or control vector under either serum starvation B. or regular culture condition C., and either were subjected to immunoblot analysis with anti-HA antibody, or labeled with BrdU and analyzed by flow cytometry and quantified (Lower left panel). Data from a representative of at least three experiments are shown.
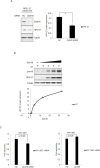
Figure 4. Dyrk1B was important for phosphorylation of p27 in HPV E7 expressing cells
A. MS/MS spectra for peptide from human p27 spanning amino acid residues VSNGpSPSLER (6-15) in HPV-16 E7 expressing epithelial cells. Inset, amino acid sequence for the peptide. Masses that show an increase of amu are marked with an asterisk. B. and C. Expression and phosphorylation of p27 in HPV-16 E7 expressing cells. p27 and phospho-p27 levels in PHK B. and RPE1 C. cells expressing E7 or control were examined by Western blot and quantified (Lower panels). C. Dyrk1B is important for p27 phosphorylation in E7 expressing cells. RPE1-E7 cells were transfected with non-specific control (NC) or Dyrk1B specific siRNAs at a final concentration of 20 nM. One set dish of cells was cultured in serum-free medium. Forty-eight hours post-transfection, cells were harvested, lysed and protein levels were analyzed by immunoblot and quantified (Lower panel). β-tubulin was used as a loading control. A representative experiment of four is shown.
Figure 5. Phosphorylation of p27 was important for S-phase entry in E7 expressing quiescent cells
A. RPE1-E7 cells were transfected with siRNA targeting p27 and plasmids encoding FLAG-tagged wild-type and mutant p27. Forty-eight hours post-transfection, cells were harvested, lysed and protein levels were analyzed by immunoblot and quantified (Lower panel). β-tubulin was used as a loading control. Cropped gels from a representative experiment are displayed. B. The above treated cells were stained with anti-BrdU antibody, counterstained with 7-AAD, and analyzed by flow cytometry and quantified (Right panel). The percentage of BrdU-positive cells was indicated. A representative experiment of three is shown.
Figure 6. Dyrk1B increased the steady-state levels of HPV-16 E7
A. RPE1-E7 cells were transfected with siRNA targeting Dyrk1B and cultured in serum free medium and Dyrk1B protein levels were analyzed by immunoblot and quantified (Right panel). β-tubulin was used as a loading control. B. RPE1 cells were transfected with plasmids encoding HPV-16 E7 and varying amounts of plasmids encoding Dyrk1B. The steady-state levels of HPV-16 E7 as well as Dyrk1B were examined by Western blot and quantified (Lower panel). β-tubulin was used as a loading control. A representative experiment of four is presented. C. RPE1-E7 cells were transfected with siRNA targeting Dyrk1B (left panel) or the plasmid encoding HA-Dyrk1B fusion and mRNA were analyzed by Real-time PCR. The graphs represent data from an average of 3 independent experiments, each done in triplicate and normalized to GAPDH levels.
Similar articles
- Cancerous inhibitor of protein phosphatase 2A contributes to human papillomavirus oncoprotein E7-induced cell proliferation via E2F1.
Zhang W, Chen H, Chen Y, Liu J, Wang X, Yu X, Chen JJ, Zhao W. Zhang W, et al. Oncotarget. 2015 Mar 10;6(7):5253-62. doi: 10.18632/oncotarget.2867. Oncotarget. 2015. PMID: 25650660 Free PMC article. - Role of WDHD1 in Human Papillomavirus-Mediated Oncogenesis Identified by Transcriptional Profiling of E7-Expressing Cells.
Zhou Y, Zhang Q, Gao G, Zhang X, Liu Y, Yuan S, Wang X, Chen JJ. Zhou Y, et al. J Virol. 2016 Jun 10;90(13):6071-6084. doi: 10.1128/JVI.00513-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099318 Free PMC article. - Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Munagala R, et al. Cancer Lett. 2015 Jan 28;356(2 Pt B):536-46. doi: 10.1016/j.canlet.2014.09.037. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304375 - A wake-up call to quiescent cancer cells - potential use of DYRK1B inhibitors in cancer therapy.
Becker W. Becker W. FEBS J. 2018 Apr;285(7):1203-1211. doi: 10.1111/febs.14347. Epub 2017 Dec 12. FEBS J. 2018. PMID: 29193696 Review. - A safe haven for cancer cells: tumor plus stroma control by DYRK1B.
Ems M, Brichkina A, Lauth M. Ems M, et al. Oncogene. 2025 Feb;44(6):341-347. doi: 10.1038/s41388-025-03275-6. Epub 2025 Jan 25. Oncogene. 2025. PMID: 39863750 Free PMC article. Review.
Cited by
- Minibrain-related kinase/dual-specificity tyrosine-regulated kinase 1B implication in stem/cancer stem cells biology.
Kokkorakis N, Gaitanou M. Kokkorakis N, et al. World J Stem Cells. 2020 Dec 26;12(12):1553-1575. doi: 10.4252/wjsc.v12.i12.1553. World J Stem Cells. 2020. PMID: 33505600 Free PMC article. Review. - Dual-Specificity, Tyrosine Phosphorylation-Regulated Kinases (DYRKs) and cdc2-Like Kinases (CLKs) in Human Disease, an Overview.
Lindberg MF, Meijer L. Lindberg MF, et al. Int J Mol Sci. 2021 Jun 3;22(11):6047. doi: 10.3390/ijms22116047. Int J Mol Sci. 2021. PMID: 34205123 Free PMC article. Review. - Interplay Between CMGC Kinases Targeting SR Proteins and Viral Replication: Splicing and Beyond.
Pastor F, Shkreta L, Chabot B, Durantel D, Salvetti A. Pastor F, et al. Front Microbiol. 2021 Mar 29;12:658721. doi: 10.3389/fmicb.2021.658721. eCollection 2021. Front Microbiol. 2021. PMID: 33854493 Free PMC article. Review. - Mirk/Dyrk1B controls ventral spinal cord development via Shh pathway.
Kokkorakis N, Douka K, Nalmpanti A, Politis PK, Zagoraiou L, Matsas R, Gaitanou M. Kokkorakis N, et al. Cell Mol Life Sci. 2024 Jan 31;81(1):70. doi: 10.1007/s00018-023-05097-9. Cell Mol Life Sci. 2024. PMID: 38294527 Free PMC article. - Cdc6 contributes to abrogating the G1 checkpoint under hypoxic conditions in HPV E7 expressing cells.
Chen H, Zhang Q, Qiao L, Fan X, Zhang W, Zhao W, Chen JJ. Chen H, et al. Sci Rep. 2017 Jun 7;7(1):2927. doi: 10.1038/s41598-017-03060-w. Sci Rep. 2017. PMID: 28592805 Free PMC article.
References
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature reviews. 2002;2:342–350. - PubMed
- Schwarz E, Freese UK, Gissman L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases