The Polycystin-1, Lipoxygenase, and α-Toxin Domain Regulates Polycystin-1 Trafficking - PubMed (original) (raw)
The Polycystin-1, Lipoxygenase, and α-Toxin Domain Regulates Polycystin-1 Trafficking
Yaoxian Xu et al. J Am Soc Nephrol. 2016 Apr.
Abstract
Mutations in polycystin-1 (PC1) give rise to autosomal dominant polycystic kidney disease, an important and common cause of kidney failure. Despite its medical importance, the function of PC1 remains poorly understood. Here, we investigated the role of the intracellular polycystin-1, lipoxygenase, and α-toxin (PLAT) signature domain of PC1 using nuclear magnetic resonance, biochemical, cellular, and in vivo functional approaches. We found that the PLAT domain targets PC1 to the plasma membrane in polarized epithelial cells by a mechanism involving the selective binding of the PLAT domain to phosphatidylserine and L-α-phosphatidylinositol-4-phosphate (PI4P) enriched in the plasma membrane. This process is regulated by protein kinase A phosphorylation of the PLAT domain, which reduces PI4P binding and recruits β-arrestins and the clathrin adaptor AP2 to trigger PC1 internalization. Our results reveal a physiological role for the PC1-PLAT domain in renal epithelial cells and suggest that phosphorylation-dependent internalization of PC1 is closely linked to its function in renal development and homeostasis.
Keywords: autosomal dominant polycystic kidney disease; genetics and development; polycystic kidney disease.
Copyright © 2016 by the American Society of Nephrology.
Figures
Figure 1.
Predicted domain structure of PC1 and sequence conservation of PLAT. (A) Domain architecture of PC1. The name of each domain is labeled in the box on the right. The position of PLAT domain (red) is in the predicted first intracellular loop. (B) Amino acid sequence alignment of PLAT from different homologs. Conserved residues are labeled with a black background. Similar residues are in a gray background. There are 24 identically conserved residues (19.4% of the total of 116 residues), which are shown as capital letters in the consensus sequence, and 89 similarly conserved residues (71.8% of the total of 116 residues), which are represented as lowercase letters in the consensus line. Eight _β_-strands are indicated by hyphens and numbered sequentially. The calcium binding site (DGD) is colored red, with key residues marked by red dots. The phosphorylation site (RNS) is labeled green, and key residues are indicated by green colons. A tyrosine sorting signal (YEIL) is labeled orange. The residues involved in PI4P binding to PLAT are labeled with a light blue background in the consensus sequence, and those binding PS are shown with a magenta background. Accession numbers are human (P98161), monkey (G7NQU9), mouse (O08852), rat (F1MAD3), dog (Q7YQK5), Xenopus (Q4G444), zebrafish (U3JAI2), and worm (Q09624 and LOV-1). The 40 human mutations reported for PLAT so far (
) are listed below: definitely, highly likely, or likely pathogenic mutations are in red, and indeterminate or likely neutral mutations are in black.
Figure 2.
PC1-PLAT binds selectively to the anionic phospholipids PS and PI4P. (A) Protein-lipid overlay assay showing lipid spots bound by (left panel) MBP-PLAT protein and (center panel) negative control using MBP protein only. (Right panel) Lipids arrayed on strip are displayed. (B) Protein-lipid bead pulldown assay showing that MBP-PLAT is pulled down by PI4P–coated agarose beads, confirming an interaction between PLAT and PI4P. Control beads were uncoated with lipid. MBP did not bind to either control or PI4P-coated beads. (C and D) Cosedimentation assays of PS and PI4P-containing liposomes with wild–type or S3164D mutant MBP-PLAT in the presence or absence of Ca2+. The bars show the mean percentages of added total protein pelleted, and the error bars represent the SEM (_n_=3). *P<0.05 versus wild-type PLAT. (E, upper panel) Recombinant MBP-PLAT is phosphorylated by PKA on S3164 but not S3132 in an in vitro kinase assay. The pathogenic mutation R3162C prevents phosphorylation of PLAT by PKA. (E, lower panel) Equal loading is shown by Coomassie blue staining. CL, cardiolipin; WT, wild type.
Figure 3.
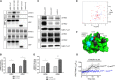
NMR analysis reveals discrete binding residues in PC1-PLAT for Ca2+, DPPS and PI4P. (A) A small region of the 15N HSQC spectrum of GB1-PLAT showing chemical shift changes (of H3161 and H3174) on addition of Ca2+. Changes were observed for residues H3137, V3138, D3155, G3156, D3157, R3158, A3159, F3160, H3161, R3162, N3163, H3174, L3176, and S3210. (B) Cartoon representation of the structure of PC1-PLAT showing the locations of residues identified as titrating with Ca2+ (red). The calcium binding loop is identified by the cluster of residues at the top of the structure. (C) A region of the 15N HSQC spectrum of GB1-PLAT showing apo-PLAT (red), calcium-loaded PLAT (blue), and calcium-loaded PLAT plus DPPS (green). DPPS removes calcium from PLAT, meaning that, where chemical shift changes occur, the green peak is usually about 70% of the way from blue to red. However, specific shift changes caused by DPPS binding (e.g., F3212; ringed in red) show a different pattern. (D) The location of the binding site for PS. The calcium loop is shown in blue, with the approximate position of the Ca2+ ion as an orange sphere; other residues affected in DPPS titrations are in red. (E) The binding site on PC1-PLAT for PI4P. Residues that move on addition of PI4P are colored red for S3164 and magenta for the others. A potential phosphate pocket is indicated by the arrow. For reference, the Ca2+ ion is shown in orange. An NMR titration with PI(4,5)P2 showed no binding (data not shown). (F) Cartoon representation of the structure of PLAT showing the position of bound calcium (brown sphere), PS (cyan/red sticks; for clarity, only one lipid chain is shown), and PI4P (green/red and orange for the phosphates) plus residues affected by the binding of PS (cyan) and PI4P (magenta/red; like in E). Residues in dark blue are broadened (by self-association) in NMR, suggesting a protein interaction site, and it is significant that the KIR3183 sequence, identified as a potential interaction site, is adjacent to it and shown in gray.
Figure 4.
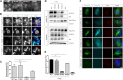
PC1–1 PLAT phosphorylation by PKA regulates its surface expression and cilia localization. (A) Fluorescence microscopy of stably expressed YFP-PLAT and YFP-PLAT mutant proteins in MDCK II cells. YFP-PLAT was consistently localized to the PM, except for the S3164D mutation, which resulted in complete loss of surface localization. (B and C) Live–cell surface labeling of PC1 in cells transfected with wild-type and S3164/R3162 FLAG-PC1-HA–tagged plasmids. Surface PC1 expression was visualized in nonpermeabilized cells by FLAG labeling; total number of PC1-expressing cells by HA staining after permeabilization are shown. PC2-CFP coexpression was visualized by epifluorescence. Cell surface labeling of PC1 was almost completely abolished in the S3164D mutant compared with the wild type or S3164A. The pathogenic mutation R3162C showed detectable but significantly reduced surface expression. Values shown are the means±SEM. Scale bar, 10 _µ_m. (D and E) Surface biotinylation of PC1 and S3164/R3162 mutants. Wild-type PC1 was biotinylated only in the presence of coexpressed PC2 and significantly reduced in the S3164D mutant. Controls included the PM transporter Na+-K+-ATPase and the ER resident protein calnexin. Lysates show equivalent expression of PC1 and PC2 in different samples. The cleaved PC1-CTF (detectable by HA) was used for quantification of surface biotinylation as previously described, because the PC1-NTF is detached under the assay conditions used., The bars indicate the ratio of biotinylated PC1 to total PC1 (HA blot) from three experiments. (F) Cilia localization of PC1 and PC2 in a normal human kidney cell line (UCL93/3) expressing the wild type or S3164/R3162 FLAG-PC1-HA. Wild-type PC1 was only detected in primary cilia when PC2-CFP was coexpressed (72% ciliated cells). There was a significant reduction in cilia expression for S3164D (6%) and S3162C (30%) but not S3164A (70%), even in the presence of PC2-CFP. An Arl13b antibody was used to label primary cilia. Scale bar, 10 _µ_m. *P<0.05; **P<0.01; ****P<0.0001.
Figure 5.
Full-length PC1 undergoes cAMP-stimulated endocytosis dependent on phosphorylation at S3164. (A and B) Live–cell surface labeling of PC1 and S3164/R3162 mutants after cAMP stimulation. Unlike wild-type PC1, PC1-S3164A did not respond to cAMP. PC1-R3162C showed a significant reduction in baseline surface expression but also, a smaller response to cAMP at 60 minutes. **P<0.01; ***P<0.001 versus baseline (serum free; _n_=3). (C and D) Surface biotinylation of wild-type FLAG-PC1-HA after stimulation with dbcAMP and forskolin. Cells were pretreated for 30 minutes with brefeldin A (BFA) to block new protein export before cAMP stimulation. A significant decrease in surface PC1 (cleaved C–terminal fragment [CTF]) was observed after a 60-minute incubation with dbCAMP and forskolin. Arrowheads indicate full-length (FL) or CTF as detected by an HA blot. The blot shown is representative of three independent experiments. *P<0.05 BFA+cAMP versus BFA (_n_=3); **P<0.01 BFA+cAMP versus control.
Figure 6.
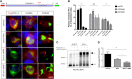
ARRB1/2 and AP2 mediate cAMP-mediated endocytosis of PC1. (A and B) Live–cell surface labeling of full-length PC1 in HEK293 cells cotransfected with scrambled-, AP2M1-, or ARRB1 1/2–specific siRNA. A significant inhibition of cAMP–stimulated PC1 endocytosis was observed with AP2M1 or ARRB1 siRNA–treated cells compared with scrambled siRNA–transfected cells. ***P<0.001. Scale bar, 10 _µ_m. (C and D) Live–cell surface labeling of full–length PC1-S3164D in HEK293 cells cotransfected with scrambled-, AP2M1-, or ARRB1 1/2–specific siRNA. PC1-S3164D surface expression was significantly increased in cells transfected with AP2M1 or ARRB1/2 siRNA compared with scrambled siRNA–transfected cells. ****P<0.0001. Scale bar, 10 _µ_m. (E and F) Surface biotinylation of PC1-S3164D in HEK293 cells showed that siRNA knockdown of AP2M1 or ARRB1/2 resulted in an increase in surface-labeled PC1 compared with scrambled siRNA controls. Arrowheads indicate full-length (FL) or cleaved C–terminal fragment (CTF) as detected by an HA blot. Controls included the PM transporter Na+-K+-ATPase and the ER resident protein calnexin. The bars show the ratio of biotinylated PC1 to total PC1 (CTF; HA blot) from three experiments. **P<0.01; ***P<0.001.
Figure 7.
PKA phosphorylation of PLAT increases its binding affinity to ARRB1/2. (A and B) MBP-PLAT pulldown assays show that PLAT can bind to ARRB1 and ARRB2, and this interaction was significantly increased by 2-fold for the S3164D-PLAT MBP fusion compared with the wild type or S3164A-PLAT. Molecular mass of MBP is 43kD, MBP-PLAT is 57kD, His-ARRB1 is 49kD, and His-ARRB2 is 46kD, respectively. *P<0.05 versus S3164A-PLAT (_n_=3). (C and D) MBP-PLAT pulldown assays after PKA phosphorylation of PLAT. PLAT binding to ARRB1 and ARRB2 was significantly increased by 1.5- to 2-fold after PKA pretreatment of MBP-PLAT. *P<0.05; ***P<0.001 versus control MBP-PLAT (_n_=3). (E) TROSY HSQC spectra of 200 _μ_M GB1-PLAT alone (blue) and in the presence of 1.5 equivalents ARRB1 (red; superimposed). Peaks with significantly reduced intensity in the presence of ARRB1 are blue. Residues K3125, G3129, R3130, H3161, R3162, G3177, Q3198, D3217, S3220, and V3234 show a clear progressive loss of intensity throughout the titration. (F) Mapping of these residues onto the surface of PLAT. The most strikingly affected residues are shown in black. Also shown are S3164 (red), the site of phosphorylation, and the region shown to interact with phosphatidyl serine (cyan), indicating that ARRB1 binds adjacent to S3164 and the membrane binding site. (G) Averaged time course of the association between PLAT-CFP and ARRB2-YFP in response to forskolin. FRET recordings are shown as normalized ratios and represent mean values±SEM (_n_=15). Addition of forskolin to a single cell increased the FRET signal by approximately 2%, with a time constant of _t_1/2=1.14 minutes. A membrane-anchored CFP (CFPm) served as a negative control.
Figure 8.
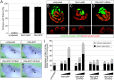
Ectopic expression of PLAT-GFP causes PKD in Xenopus embryos. (A) Xenopus embryos injected radially with a total of 3.2 pmol Pkd1-sMO or 8 ng Plat-GFP mRNA as well as uninjected control embryos were analyzed at stage 42 by morphology. Bar diagram shows the percentage of embryos with edema as a sign of kidney dysfunction. Error bar corresponds to SD of multiple experiments. (B–D) Whole–mount immunofluorescence analysis with 3G8 (green) and 4A6 (red) of the embryos in A to visualize proximal tubules as well as distal tubules and duct, respectively. Upper panels show three-dimensional reconstruction of z stacks, whereas lower panels are cross-sections through individual proximal tubules. (E–I) Xenopus embryos were injected at the four-cell stage into a single blastomere with increasing amounts (0.5, 1.0, and 2.0 ng) of wild-type Plat-GFP, Plat-GFP(S3164D), Plat-GFP(S3164A), and Plat-GFP(R3162C). Expression of Nbc1 in the DT1 distal tubular segment was examined by whole–mount in situ hybridization comparing the injected and the contralateral noninjected pronephri at stage 39. E–H show representative embryos (Nbc1 staining in the DT1 segment is indicated by arrows). (I) Quantification of three independent experiments depicting the percentages of embryos with absent and reduced Nbc1 expressions. The total number of embryos analyzed is indicated above the bars.
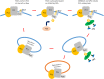
Figure 9.
Binding of phosphorylated PLAT to ARRB1/2 drives internalisation of a PC1/PC2 complex. PLAT binding to PI4P/PS at the inner leaflet of the PM is required for its surface localization and/or retention. As previously reported and confirmed in this study, binding to PC2 is required for PC1 trafficking to the cell surface. PKA phosphorylation at S3164 leads to a reduction in PI4P binding and the recruitment of ARRB1/2 and AP2. This results in the internalization of PC1 into endocytic vesicles from the PM and their likely trafficking into early endosomes for recycling and signaling or lysosomes for degradation.
Similar articles
- Backbone assignment and secondary structure of the PLAT domain of human polycystin-1.
Xu Y, Ong AC, Williamson MP, Hounslow AM. Xu Y, et al. Biomol NMR Assign. 2015 Oct;9(2):369-73. doi: 10.1007/s12104-015-9612-4. Epub 2015 May 6. Biomol NMR Assign. 2015. PMID: 25943267 - Protein phosphatase 1α interacts with a novel ciliary targeting sequence of polycystin-1 and regulates polycystin-1 trafficking.
Luo C, Wu M, Su X, Yu F, Brautigan DL, Chen J, Zhou J. Luo C, et al. FASEB J. 2019 Sep;33(9):9945-9958. doi: 10.1096/fj.201900338R. Epub 2019 Jun 3. FASEB J. 2019. PMID: 31157564 Free PMC article. - Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2.
Casuscelli J, Schmidt S, DeGray B, Petri ET, Celić A, Folta-Stogniew E, Ehrlich BE, Boggon TJ. Casuscelli J, et al. Am J Physiol Renal Physiol. 2009 Nov;297(5):F1310-5. doi: 10.1152/ajprenal.00412.2009. Epub 2009 Sep 2. Am J Physiol Renal Physiol. 2009. PMID: 19726544 Free PMC article. - Regulation of ciliary trafficking of polycystin-2 and the pathogenesis of autosomal dominant polycystic kidney disease.
Cai Y, Tang Z. Cai Y, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010 Feb;35(2):93-9. doi: 10.3969/j.issn.1672-7347.2010.02.001. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20197605 Review. - The Role of G-Protein-Coupled Receptor Proteolysis Site Cleavage of Polycystin-1 in Renal Physiology and Polycystic Kidney Disease.
Trudel M, Yao Q, Qian F. Trudel M, et al. Cells. 2016 Jan 21;5(1):3. doi: 10.3390/cells5010003. Cells. 2016. PMID: 26805887 Free PMC article. Review.
Cited by
- Polycystin 1 loss of function is directly linked to an imbalance in G-protein signaling in the kidney.
Zhang B, Tran U, Wessely O. Zhang B, et al. Development. 2018 Mar 22;145(6):dev158931. doi: 10.1242/dev.158931. Development. 2018. PMID: 29530879 Free PMC article. - Protein salvage and repurposing in evolution: Phospholipase D toxins are stabilized by a remodeled scrap of a membrane association domain.
Cordes MHJ, Sundman AK, Fox HC, Binford GJ. Cordes MHJ, et al. Protein Sci. 2023 Jul;32(7):e4701. doi: 10.1002/pro.4701. Protein Sci. 2023. PMID: 37313620 Free PMC article. - Characterization of Epidermal Lipoxygenase Expression in Normal Human Skin and Tissue-Engineered Skin Substitutes.
Simard-Bisson C, Parent LA, Moulin VJ, Fruteau de Laclos B. Simard-Bisson C, et al. J Histochem Cytochem. 2018 Nov;66(11):813-824. doi: 10.1369/0022155418788117. Epub 2018 Jul 9. J Histochem Cytochem. 2018. PMID: 29985723 Free PMC article. - Adhesion GPCRs as a paradigm for understanding polycystin-1 G protein regulation.
Maser RL, Calvet JP. Maser RL, et al. Cell Signal. 2020 Aug;72:109637. doi: 10.1016/j.cellsig.2020.109637. Epub 2020 Apr 16. Cell Signal. 2020. PMID: 32305667 Free PMC article. Review. - Polycystic kidney disease.
Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Bergmann C, et al. Nat Rev Dis Primers. 2018 Dec 6;4(1):50. doi: 10.1038/s41572-018-0047-y. Nat Rev Dis Primers. 2018. PMID: 30523303 Free PMC article. Review.
References
- Ong AC, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 - PubMed
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 - PubMed
- Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM: Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum Mol Genet 9: 1641–1649, 2000 - PubMed
- Streets AJ, Newby LJ, O’Hare MJ, Bukanov NO, Ibraghimov-Beskrovnaya O, Ong AC: Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J Am Soc Nephrol 14: 1804–1815, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Medical Research Council/United Kingdom
- R01-DK102495/DK/NIDDK NIH HHS/United States
- R01-DK087688/DK/NIDDK NIH HHS/United States
- R01-DK080745-05/DK/NIDDK NIH HHS/United States
- R01 DK080745/DK/NIDDK NIH HHS/United States
- R01 DK102495/DK/NIDDK NIH HHS/United States
- R01 DK087688/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous