In situ structural analysis of Golgi intracisternal protein arrays - PubMed (original) (raw)
In situ structural analysis of Golgi intracisternal protein arrays
Benjamin D Engel et al. Proc Natl Acad Sci U S A. 2015.
Abstract
We acquired molecular-resolution structures of the Golgi within its native cellular environment. Vitreous Chlamydomonas cells were thinned by cryo-focused ion beam milling and then visualized by cryo-electron tomography. These tomograms revealed structures within the Golgi cisternae that have not been seen before. Narrow trans-Golgi lumina were spanned by asymmetric membrane-associated protein arrays that had ∼6-nm lateral periodicity. Subtomogram averaging showed that the arrays may determine the narrow central spacing of the trans-Golgi cisternae through zipper-like interactions, thereby forcing cargo to the trans-Golgi periphery. Additionally, we observed dense granular aggregates within cisternae and intracisternal filament bundles associated with trans-Golgi buds. These native in situ structures provide new molecular insights into Golgi architecture and function.
Keywords: Chlamydomonas; Golgi; cryo-electron tomography; focused ion beam; glycosyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
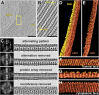
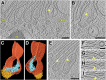
Intracisternal membrane-associated protein arrays in the trans-Golgi. (A) Overview and (B) close-up tomogram slices showing a Golgi with an intracisternal protein array within the third cisterna from the left. B is enlarged from the box in A. tsm, trans-side membrane; csm, cis-side membrane. (C) Fourier analysis of the protein array. For each row, the frequency image (power spectrum) is on the Left, and the corresponding real-space image is on the Right. (Top row) Original image, rotated from B. (Second row) Masking the 11.8-nm peak in the power spectrum removes the alternation between long and short proteins projecting into the lumen. (Third row) Masking both the 11.8-nm and 5.9-nm peaks removes the protein array altogether. (Bottom row) Masking the central peak removes the cisterna’s lipid bilayers, but densities remain within the cis-side bilayer region that may be the array’s transmembrane domains. (D_–_H) Threshold-based segmentation of the cisterna containing the protein array. (D) Segmentation in the same orientation as B, separated into the cisterna’s trans-side (yellow) and cis-side (dark orange). (E) Segmentation with the trans-side removed, revealing ordered rows of luminal projections. (F) Orthogonal view of the cis-side’s luminal face. (G) Side view showing alternating rows of small projections that do not contact the trans-side and long projections with multiple contact sites (yellow). (H) View of the array’s cytoplasmic face, with ridges that correspond to the positions of the luminal projections. (Scale bars, 200 nm in A; 50 nm in B.)
Fig. S1.
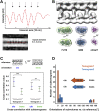
(A) Subtomogram average of extracted array structures from tomograms 2–8 (194 subvolumes), which were not used to generate the structure in Fig. 3. Mass is shown in white. A linescan through the central xy slice reveals an average lateral repeat in the range of 5.5–6.8 nm. This is consistent with the 5.9-nm repeat of the highly ordered array in tomogram 1 (Figs. 1 and 3). Arrows: intensity peak positions from the linescan corresponding to luminal densities in the average. (B) The subtomogram average of the array in tomogram 1 (Fig. 3) is displayed to scale with the crystal structures of three glycosyltransferase luminal catalytic domains, fucosyltransferase FUT6 (PDB: 2NZW) (78), mannosidase GMII (PDB: 1HTY) (79), and galactosyltransferase α3GaIT (PDB: 1G8O) (80). These structures are comparable in size with the luminal densities identified in the array average. (C) Cross-correlation values were calculated by comparing the reference structure (from Fig. 3) in the cis and trans orientations to the individual subtomograms from tomogram 1 (containing the highly ordered array, Figs. 1 and 3) and tomogram 2 (another high quality tomogram, Fig. 2). The arrays from both tomograms show a high degree of asymmetry, favoring the cis orientation- tomogram 1 at a significance level of 0.1% (***) and tomogram 2 at 1% (**). Error bars, SD. (D) Histograms showing the final orientations of each subvolume relative to the cis reference used for alignment; 88.4% of the arrays from tomogram 1 (red) and 80.6% of the arrays from tomogram 2 (blue) were in the cis orientation (larger projections from the cis-sides of the cisternae), the same orientation as the highly ordered array shown in Figs. 1 and 3.
Fig. 2.
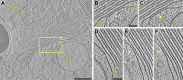
Protein arrays likely maintain the trans-Golgi’s narrow luminal spacing. (A) Overview and (B and C) two sequential slices through a tomogram showing a protein array (arrows) that is exclusively located where the trans-most cisterna’s membranes are closely apposed. B and C correspond to the box in A. (D_–_F) Three sequential slices through another tomogram showing a protein array that is only found where the trans-most cisterna’s membranes are closely apposed. tsm, trans-side membrane, csm, cis-side membrane. (Scale bars, 200 nm in A; 50 nm in B_–_F.)
Fig. 3.
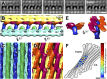
Subtomogram average and localization of the intracisternal protein arrays. (A_–_E) Symmetrized subtomogram average calculated from the array in Fig. 1. (A) Sequential side view slices every 3.4 nm through the average, showing the array’s periodicity and asymmetric structure. Mass is shown in white. (B) Segmentation of the average into the following regions: cis-side bilayer and embedded proteins (cyan), trans-side bilayer and embedded proteins (yellow), long 5.5-nm and short 3.5-nm luminal projections from the cis-side bilayer (blue and green, respectively), and 2.5-nm luminal projections from the trans-side bilayer (magenta and orange). (C) View of the cis-side’s luminal surface, rotated 90° from B, showing alternating rows of short and long projections. (D) View of the trans-side’s luminal surface, rotated 90° from B, showing an interlocking network of projections. Asterisks in C and D: contact sites between the cis-side and trans-side projections. The periodicity of contacts along the rows of projections is 10.3 nm. (E) Side views showing how the rows of long cis-side projections form _y_-shaped zipper-like interactions with the rows of trans-side projections. (F) Heat map displaying the density of protein arrays (gradient of gray to red, determined by template matching) within the Golgi from Fig. 1 (black outlines). (Scale bars, 10 nm in A; 50 nm in F.)
Fig. 4.
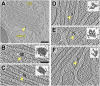
Intracisternal filament bundles near COPI buds in the trans-Golgi. (A and B) Two sequential slices through a tomogram showing a filament bundle (arrows) within the trans-most cisterna, close to a coated bud. ac, an acidocalcisome containing a dense aggregate of polyphosphate filaments. In B, an actin filament can be seen below the cisterna, along with a vesicle surrounding a complex that resembles a 20S proteasome. (C and D) Segmentation of the cisterna from A and B showing the Golgi membrane (dark orange), the coated bud (yellow), and the filament bundle (blue). The view in D is flipped 180° from C. (E) A slice from a second tomogram showing filament bundles within the last two trans-Golgi cisterna, close to coated buds. Cis-Golgi is toward the left, trans-Golgi is toward the right. (F_–_I) Four sequential slices from a third tomogram showing a filament bundle within the terminal cisterna of the trans-Golgi. The bundle is adjacent to a coated bud that is undergoing scission to become a vesicle. Cis-Golgi is up, trans-Golgi is down. This tomogram was acquired with a Volta phase plate (68) to enhance contrast. (Scale bars, 100 nm.)
Fig. 5.
Dark aggregates within Golgi cisterna. (A) Overview slice from a tomogram showing a Golgi with a dark intracisternal aggregate (arrow). A COPII bud can be seen emanating from the ER. This is a more peripheral Golgi section compared with Figs. 1 and 2. (B_–_F) Slices from several tomograms showing close-ups of intracisternal aggregates and corresponding segmentations in the same orientations (Insets). B is a magnified view of the aggregate in A. (Scale bars, 200 nm in A; 50 nm in B_–_F.)
Fig. 6.
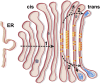
Model depicting how the intracisternal structures identified in this study may relate to traffic through the Golgi. Protein arrays (yellow and orange triangles), filament bundles (blue), and granular aggregates (dark gray) are illustrated alongside the proposed route of cargo transit (dashed arrows). (1) The aggregates likely proceed through the Golgi by cisternal maturation, although multiple transport mechanisms are possible for smaller cargo. (2) In the trans-Golgi, the protein arrays exclude cargo from the centers of cisternae, promoting cargo exit from the periphery.
Similar articles
- The structure of the COPI coat determined within the cell.
Bykov YS, Schaffer M, Dodonova SO, Albert S, Plitzko JM, Baumeister W, Engel BD, Briggs JA. Bykov YS, et al. Elife. 2017 Nov 17;6:e32493. doi: 10.7554/eLife.32493. Elife. 2017. PMID: 29148969 Free PMC article. - In situ localization of N and C termini of subunits of the flagellar nexin-dynein regulatory complex (N-DRC) using SNAP tag and cryo-electron tomography.
Song K, Awata J, Tritschler D, Bower R, Witman GB, Porter ME, Nicastro D. Song K, et al. J Biol Chem. 2015 Feb 27;290(9):5341-53. doi: 10.1074/jbc.M114.626556. Epub 2015 Jan 6. J Biol Chem. 2015. PMID: 25564608 Free PMC article. - Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography.
Engel BD, Schaffer M, Kuhn Cuellar L, Villa E, Plitzko JM, Baumeister W. Engel BD, et al. Elife. 2015 Jan 13;4:e04889. doi: 10.7554/eLife.04889. Elife. 2015. PMID: 25584625 Free PMC article. - Unveiling the cryo-EM structure of retromer.
Chandra M, Kendall AK, Jackson LP. Chandra M, et al. Biochem Soc Trans. 2020 Oct 30;48(5):2261-2272. doi: 10.1042/BST20200552. Biochem Soc Trans. 2020. PMID: 33125482 Free PMC article. Review. - Cryo-Electron Tomography and Subtomogram Averaging.
Wan W, Briggs JA. Wan W, et al. Methods Enzymol. 2016;579:329-67. doi: 10.1016/bs.mie.2016.04.014. Epub 2016 Jun 22. Methods Enzymol. 2016. PMID: 27572733 Review.
Cited by
- Preparing Arabidopsis thaliana root protoplasts for cryo electron tomography.
Sanchez Carrillo IB, Hoffmann PC, Barff T, Beck M, Germain H. Sanchez Carrillo IB, et al. Front Plant Sci. 2023 Sep 22;14:1261180. doi: 10.3389/fpls.2023.1261180. eCollection 2023. Front Plant Sci. 2023. PMID: 37810374 Free PMC article. - Heuristic Modeling and 3D Stereoscopic Visualization of a Chlamydomonas reinhardtii Cell.
Biere N, Ghaffar M, Doebbe A, Jäger D, Rothe N, Friedrich BM, Hofestädt R, Schreiber F, Kruse O, Sommer B. Biere N, et al. J Integr Bioinform. 2018 Jul 11;15(2):20180003. doi: 10.1515/jib-2018-0003. J Integr Bioinform. 2018. PMID: 30001212 Free PMC article. - DNA origami signposts for identifying proteins on cell membranes by electron cryotomography.
Silvester E, Vollmer B, Pražák V, Vasishtan D, Machala EA, Whittle C, Black S, Bath J, Turberfield AJ, Grünewald K, Baker LA. Silvester E, et al. Cell. 2021 Feb 18;184(4):1110-1121.e16. doi: 10.1016/j.cell.2021.01.033. Cell. 2021. PMID: 33606980 Free PMC article. - Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis.
Zhang Y, Nikolovski N, Sorieul M, Vellosillo T, McFarlane HE, Dupree R, Kesten C, Schneider R, Driemeier C, Lathe R, Lampugnani E, Yu X, Ivakov A, Doblin MS, Mortimer JC, Brown SP, Persson S, Dupree P. Zhang Y, et al. Nat Commun. 2016 Jun 9;7:11656. doi: 10.1038/ncomms11656. Nat Commun. 2016. PMID: 27277162 Free PMC article. - Proteasomes tether to two distinct sites at the nuclear pore complex.
Albert S, Schaffer M, Beck F, Mosalaganti S, Asano S, Thomas HF, Plitzko JM, Beck M, Baumeister W, Engel BD. Albert S, et al. Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):13726-13731. doi: 10.1073/pnas.1716305114. Epub 2017 Dec 11. Proc Natl Acad Sci U S A. 2017. PMID: 29229809 Free PMC article.
References
- Medalia O, et al. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science. 2002;298(5596):1209–1213. - PubMed
- Asano S, et al. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science. 2015;347(6220):439–442. - PubMed
- Marko M, Hsieh C, Schalek R, Frank J, Mannella C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat Methods. 2007;4(3):215–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources