Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions - PubMed (original) (raw)
Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions
Wenxuan Liu et al. Elife. 2015.
Abstract
Skeletal muscle maintenance depends on motor innervation at neuromuscular junctions (NMJs). Multiple mechanisms contribute to NMJ repair and maintenance; however muscle stem cells (satellite cells, SCs), are deemed to have little impact on these processes. Therefore, the applicability of SC studies to attenuate muscle loss due to NMJ deterioration as observed in neuromuscular diseases and aging is ambiguous. We employed mice with an inducible Cre, and conditionally expressed DTA to deplete or GFP to track SCs. We found SC depletion exacerbated muscle atrophy and type transitions connected to neuromuscular disruption. Also, elevated fibrosis and further declines in force generation were specific to SC depletion and neuromuscular disruption. Fate analysis revealed SC activity near regenerating NMJs. Moreover, SC depletion aggravated deficits in reinnervation and post-synaptic morphology at regenerating NMJs. Therefore, our results propose a mechanism whereby further NMJ and skeletal muscle decline ensues upon SC depletion and neuromuscular disruption.
Keywords: aging; cell biology; denervation; developmental biology; motor neuron; mouse; sarcopenia; satellite cell; stem cells; synapse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
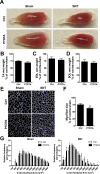
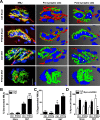
Figure 1.. Depletion of Pax7+ SCs in P7DTA skeletal muscles.
(A) Scheme demonstrating time of Tmx treatment, Sciatic nerve transection (SNT) surgery, and harvest of tissue. Representative images of TA transverse sections, stained with anti-Pax7 (red), anti-Laminin (white) and DAPI (blue). Red arrowheads indicate Pax7+ cells. (B) Quantification of Pax7+ satellite cell (SC) number from Ctrl and P7DTA TA muscles 6 weeks after sham or SNT surgery. N = 3 mice, 3 sections/mouse, 6 fields/ section. Scale bar = 50 μm. *p < 0.05 compared to Ctrl, **p < 0.05 compared to Ctrl sham, ANOVA/Bonferroni multiple comparisons test. DOI:
http://dx.doi.org/10.7554/eLife.09221.003
Figure 2.. SC depletion exacerbates neuromuscular disruption induced myofiber atrophy.
(A) Representative images of TA muscles. Scale bar = 5 mm. (B–D) Quantification of (B) TA (C) EDL and (D) Soleus (SOL) muscle wet weight after SNT as a percentage of contralateral sham. N = 6 for Ctrl and 8 for P7DTA. (E) Representative TA sections stained with anti-Laminin (white) and DAPI (blue). Scale bar = 50 μm. (F) Quantification of TA myofiber size as a percentage of contralateral sham. (G) Histograms of TA myofiber size distributions. N = 4 mice,
>
1000 myofibers/mouse. *p < 0.05, t-tests. DOI:
http://dx.doi.org/10.7554/eLife.09221.004
Figure 2—figure supplement 1.. Retention of myofibers and modest loss of myonuclei 6 weeks after SNT.
(A) Representative images of single isolated myofibers from EDL muscles (fixed prior to isolation from lower limbs), stained with DAPI. (B, C) Quantification of EDL (B) myofiber number and (C) myonuclei number per 500 μm. N = 4 mice, 32 myofibers/mouse, *p < 0.05 compared to Ctrl-sham, **p < 0.05 compared to Ctrl-sham, Ctrl-SNT, and P7DTA-sham ANOVA/Bonferroni multiple comparisons test and t-tests. DOI:
http://dx.doi.org/10.7554/eLife.09221.005
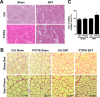
Figure 3.. SC depletion induces connective tissue accumulation in skeletal muscles after neuromuscular disruption.
(A) Representative images of TA sections stained with H&E; scale bar = 100 μm, (B) Representative images of TA sections stained Sirius Red and pseudocolor images generated by VisioPharm software; numbers indicate myofiber connective tissue (MCT) (red) content in each representative image; scale bar = 50 μm. (C) Quantification of MCT content in TA muscles. N = 4 mice. *p < 0.05 compared to Ctrl-sham, P7DTA-sham and Ctrl-SNT, ANOVA/Bonferroni multiple comparisons test. DOI:
http://dx.doi.org/10.7554/eLife.09221.006
Figure 4.. SC depletion aggravates myofiber type transitions connected to neuromuscular disruption.
(A) Representative images of Ctrl and P7DTA inner TA/EDL muscle regions 6 weeks after sham and SNT surgery stained as indicated with anti-MyHC IIX, all MyHCs except IIX, MyHC IIA, MyHC IIB and MyHC I. Also depicted in Merge IIX+/IIX-, MyHC IIB/IIA and MyHC I labeled images are stains for anti-Laminin (white) and DAPI (blue). (B) Quantification of type IIX pure (green only) and hybrid (green and red, labeled with asterisks) myofiber percentages. (C–E) Quantification of (C) Type IIB (D) Type IIA and (E) Type I fiber percentage. N = 4 mice, 3 sections/mouse, 3 fields/section. Scale bar = 50 μm. *p < 0.05 compared to Ctrl-sham and P7DTA-sham, **p < 0.05 compared to Ctrl sham, P7DTA-sham and Ctrl-SNT, ANOVA/Bonferroni multiple comparisons test. DOI:
http://dx.doi.org/10.7554/eLife.09221.007
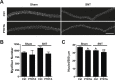
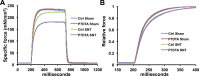
Figure 5.. SC depletion leads to declines in force generation of skeletal muscles after neuromuscular disruption.
(A) Average time to peak tension (TTP) during 150 Hz stimulation in EDL muscles. *p < 0.05 compared to Ctrl and P7DTA sham. ANOVA/Bonferroni multiple comparisons test, N = 4–6. (B) Absolute and (C) Specific force frequency curves for Ctrl and P7DTA EDL muscles 6 weeks after SNT surgery. *p < 0.05 compared to Ctrl SNT at indicated frequency, t-tests, N = 4–6 mice. DOI:
http://dx.doi.org/10.7554/eLife.09221.008
Figure 5—figure supplement 1.. Reduced contractile force and slowed force development in EDL muscles following SNT.
(A). Representative traces for specific force from in vitro muscle contraction measurements in EDL muscles stimulated at 150hz for 500ms. (B). Same traces as (A) but normalized to the corresponding peak and expanded for the first 200ms of stimulation to show the delayed force development in muscles following SNT. DOI:
http://dx.doi.org/10.7554/eLife.09221.009
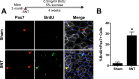
Figure 6.. Limited SC proliferation in skeletal muscles upon neuromuscular disruption.
(A) Strategy to BrdU label SCs in adult mice 4 weeks after sham or SNT surgery and representative TA sections stained with anti-Pax7 (red), anti-BrdU (green) and anti-Laminin (white). Red arrowheads indicate Pax7+ cell; green arrowhead indicates BrdU + cell; yellow arrowhead indicates BrdU+/Pax7+ cell. (B) Quantification of BrdU+/Pax7+ percentage. N = 3 mice, 3 sections/mouse, 6 fields of view/section. *p < 0.05, t-tests. DOI:
http://dx.doi.org/10.7554/eLife.09221.010
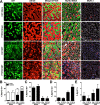
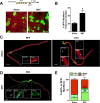
Figure 7.. Neuromuscular disruption stimulates SC contribution in the vicinity of NMJs.
(A) Scheme demonstrating time of tamoxifen treatment, SNT surgery, and harvest of tissue. Representative images to examine GFP label in myofibers and Pax7+ SCs of P7mTmG skeletal muscles 4 weeks after sham and SNT surgery. (B) Quantification of the percentage of GFP + myofibers from midbelly of EDL muscles 4 weeks after sham or SNT surgery. Scale bar = 50 μm. N = 3 mice, 3 sections/mouse, 6 fields/section. *p < 0.05, t-tests. (C, D) Representative images of single isolated P7mTmG EDL myofibers with no GFP (RFP), GFP at ends (End) or GFP in middle portions where neuromuscular junctions (NMJs) are located (Mid) after (C) sham or (D) SNT surgery. Magnified inset images show SCs (Pax7+) or NMJs (Btx, AChRs). Scale bar for myofibers = 200 μm for inset = 25 μm. (E) Quantification of GFP + fiber percentage and distribution. Note a higher percentage of myofibers after SNT express GFP primarily in the Mid regions, the location of NMJs. N = 4 mice, 25 myofibers examined per mouse. *p < 0.05 for all GFP + groups, **p < 0.05 for Mid GFP, t-tests. DOI:
http://dx.doi.org/10.7554/eLife.09221.011
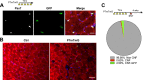
Figure 7—figure supplement 1.. P7mTmG myofibers are not GFP + when examined immediately after Tmx administration.
(A) Scheme demonstrating time of Tmx treatment, SNT surgery, and harvest of tissue. Representative images of adult P7mTmG TA muscle, taken 24 hr after Tmx treatment, sections stained for anti-GFP and anti-Pax7. (B) Images of Ctrl and P7mTmG TA muscle sections, note the lack of GFP + myofibers. (C) Proportion of central nucleated myofibers (CNF) and GFP + CNFs after SNT, 3875 myofibers examined. DOI:
http://dx.doi.org/10.7554/eLife.09221.012
Figure 8.. Reductions in NMJ reinnervation, post-synaptic morphology, and post-synaptic myonuclei in SC depleted skeletal muscle.
(A) Representative confocal IF images and 3-D Amira based reconstructions of Ctrl and P7DTA NMJs 6 weeks after sham or SNT surgery, stained for post-synaptic (AChRs labeled with Btx, green), pre-synaptic markers (SV2, Syt-2, neurofilament, red) and myonuclei (DAPI, blue). Post-synaptic myonuclei are indicated with asterisks. (B) Quantification of NMJ reinnervation: partially dennervated (Part) and fully denervated (Full). (C) Quantification of degenerated NMJs based on post-synaptic morphology. (D) Percentage distribution of NMJs based on number of post-synaptic myonuclei. Scale bar = 10 μm. N = 4 mice, 20 NMJs/mouse. *p < 0.05 compared to Ctrl and P7DTA sham, **p < 0.05 compared to Ctrl-sham, P7DTA-sham and Ctrl-SNT, ANOVA/Bonferroni multiple comparisons test. DOI:
http://dx.doi.org/10.7554/eLife.09221.013
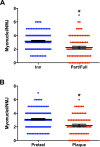
Figure 8—figure supplement 1.. Loss of post-synaptic myonuclei is a feature of NMJ degeneration.
(A, B) Quantification of Ctrl NMJ post-synaptic myonuclei: (A) Comparison between denervated and reinnervated NMJs; (B) Comparison between pretzel-like and plaque-like (degenerated) NMJs based on their post-synaptic AChR apparatus shape. DOI:
http://dx.doi.org/10.7554/eLife.09221.014
Similar articles
- Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration.
Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV. Liu W, et al. Elife. 2017 Jun 6;6:e26464. doi: 10.7554/eLife.26464. Elife. 2017. PMID: 28583253 Free PMC article. - The Composition, Development, and Regeneration of Neuromuscular Junctions.
Liu W, Chakkalakal JV. Liu W, et al. Curr Top Dev Biol. 2018;126:99-124. doi: 10.1016/bs.ctdb.2017.08.005. Epub 2017 Nov 10. Curr Top Dev Biol. 2018. PMID: 29305005 Review. - Castration induces satellite cell activation that contributes to skeletal muscle maintenance.
Klose A, Liu W, Paris ND, Forman S, Krolewski JJ, Nastiuk KL, Chakkalakal JV. Klose A, et al. JCSM Rapid Commun. 2018;1(1):e00040. JCSM Rapid Commun. 2018. PMID: 29782610 Free PMC article. - Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury.
Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ. Kang H, et al. J Neurosci. 2014 Apr 30;34(18):6323-33. doi: 10.1523/JNEUROSCI.4673-13.2014. J Neurosci. 2014. PMID: 24790203 Free PMC article. - The role of semaphorin3A in myogenic regeneration and the formation of functional neuromuscular junctions on new fibres.
Anderson JE, Do MQ, Daneshvar N, Suzuki T, Dort J, Mizunoya W, Tatsumi R. Anderson JE, et al. Biol Rev Camb Philos Soc. 2017 Aug;92(3):1389-1405. doi: 10.1111/brv.12286. Epub 2016 Jun 13. Biol Rev Camb Philos Soc. 2017. PMID: 27296513 Review.
Cited by
- Therapeutics Targeting Skeletal Muscle in Amyotrophic Lateral Sclerosis.
Gao J, Sterling E, Hankin R, Sikal A, Yao Y. Gao J, et al. Biomolecules. 2024 Jul 22;14(7):878. doi: 10.3390/biom14070878. Biomolecules. 2024. PMID: 39062592 Free PMC article. Review. - We need to talk-how muscle stem cells communicate.
Majchrzak K, Hentschel E, Hönzke K, Geithe C, von Maltzahn J. Majchrzak K, et al. Front Cell Dev Biol. 2024 Jul 10;12:1378548. doi: 10.3389/fcell.2024.1378548. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050890 Free PMC article. Review. - Skeletal muscle dysfunction in amyotrophic lateral sclerosis: a mitochondrial perspective and therapeutic approaches.
Kubat GB, Picone P. Kubat GB, et al. Neurol Sci. 2024 Sep;45(9):4121-4131. doi: 10.1007/s10072-024-07508-6. Epub 2024 Apr 27. Neurol Sci. 2024. PMID: 38676818 Free PMC article. Review. - Distinct transcriptomic profile of satellite cells contributes to preservation of neuromuscular junctions in extraocular muscles of ALS mice.
Li A, Yi J, Li X, Dong L, Ostrow LW, Ma J, Zhou J. Li A, et al. Elife. 2024 Apr 25;12:RP92644. doi: 10.7554/eLife.92644. Elife. 2024. PMID: 38661532 Free PMC article. - Denervation alters the secretome of myofibers and thereby affects muscle stem cell lineage progression and functionality.
Henze H, Hüttner SS, Koch P, Schüler SC, Groth M, von Eyss B, von Maltzahn J. Henze H, et al. NPJ Regen Med. 2024 Mar 1;9(1):10. doi: 10.1038/s41536-024-00353-3. NPJ Regen Med. 2024. PMID: 38424446 Free PMC article.
References
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990;109:943–952. - PubMed
Publication types
MeSH terms
Grants and funding
- 1S10RR027340-01/RR/NCRR NIH HHS/United States
- C026877/PHS HHS/United States
- AR059646/AR/NIAMS NIH HHS/United States
- AG051456/AG/NIA NIH HHS/United States
- R01 AG051456/AG/NIA NIH HHS/United States
- R01 AR059646/AR/NIAMS NIH HHS/United States
- S10 RR027340/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous