Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils - PubMed (original) (raw)
Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils
Laura M Castellano et al. Biol Open. 2015.
Abstract
Semen harbors amyloid fibrils formed by proteolytic fragments of prostatic acid phosphatase (PAP248-286 and PAP85-120) and semenogelins (SEM1 and SEM2) that potently enhance HIV infectivity. Amyloid but not soluble forms of these peptides enhance HIV infection. Thus, agents that remodel these amyloid fibrils could prevent HIV transmission. Here, we confirm that the green tea polyphenol, epigallocatechin-3-gallate (EGCG), slowly remodels fibrils formed by PAP248-286 termed SEVI (semen derived enhancer of viral infection) and also exerts a direct anti-viral effect. We elucidate for the first time that EGCG remodels PAP85-120, SEM1(45-107), and SEM2(49-107) fibrils more rapidly than SEVI fibrils. We establish EGCG as the first small molecule that can remodel all four classes of seminal amyloid. The combined anti-amyloid and anti-viral properties of EGCG could have utility in preventing HIV transmission.
Keywords: EGCG; HIV infectivity; PAP85-120; SEM1; SEM2; SEVI.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests
The authors declare no competing or financial interests.
Figures
Fig. 1.
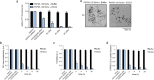
EGCG slowly remodels SEVI fibrils into non-amyloid structures. (A) Preformed SEVI fibrils (20 µM) were incubated with buffer or EGCG (200 µM) for 0–24 h. Fibril integrity was assessed by ThT fluorescence. Values represent means±s.e.m. (_n_=4). (B) Transmission electron micrographs of SEVI fibrils incubated with buffer or EGCG for 2 h or 24 h. Scale bar: 2 µm. (C) SEVI fibrils (20 µM) were incubated with buffer for 6 h or 24 h or EGCG (200 µM) for 6 h or 24 h, and the resulting products were used to seed soluble PAP248-286 (1 mM, 0.1% fibril seed) fibrillization. Buffer conditions lacking fibril seed were included. Fibril assembly was monitored by ThT fluorescence. Values represent means±s.e.m. (_n_=4). (D-F) Preformed SEVI fibrils (20 µM) were incubated with buffer or EGCG (200 µM) for 0–24 h. Fibril integrity was assessed by anti-amyloid (OC) immunoreactivity (D), turbidity (E), or sedimentation analysis (F). Values represent means±s.e.m. (_n_=3).
Fig. 2.
EGCG rapidly remodels PAP85-120 amyloid fibrils. (A-D) PAP85-120 fibrils (20 µM) were incubated with buffer or EGCG (200 µM) for 0−24 h. Fibril integrity was assessed by measuring: ThT fluorescence intensity (A), OC immunoreactivity (B), turbidity (C), or sedimentation analysis (D). Values represent means±s.e.m. (_n_=3). (E) Transmission electron micrographs of PAP85-120 fibrils incubated with buffer (untreated) or EGCG for 6 h. Scale bar: 500 nm.
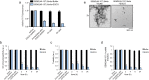
Fig. 3.
EGCG rapidly remodels SEM1(45-107) fibrils. (A) SEM1(45-107) fibrils (20 µM) were incubated with buffer or EGCG (200 µM) for 0–24 h. Fibril integrity was assessed by measuring: ThT fluorescence intensity (A), OC immunoreactivity (B), turbidity (C), or sedimentation analysis (D). Values represent means±s.e.m. (_n_=3). (E) Transmission electron micrographs of SEM1(45-107) fibrils incubated with buffer or EGCG for 2 h. Scale bar: 500 nm.
Fig. 4.
EGCG rapidly remodels SEM2(49-107) fibrils. (A) SEM2(49-107) fibrils (20 µM) were incubated with buffer or EGCG (200 µM) for 0–24 h. Fibril integrity was assessed by measuring: ThT fluorescence intensity (A), OC immunoreactivity (B), turbidity (C), or sedimentation analysis (D). Values represent means±s.e.m. (_n_=3). (E) Transmission electron micrographs of SEM2(49-107) fibrils incubated with buffer or EGCG for 2 h. Scale bar: 500 nm.
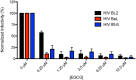
Fig. 5.
EGCG inhibits HIV infectivity in cell culture. TZM-bl cells were infected with three HIV-1 strains (BL2, BaL, and 89.6) in the presence of the indicated concentrations of EGCG (final concentration in cell culture). Infectivity was monitored by measuring luciferase activity in the cell cultures. Values represent means±s.e.m. (_n_=3).
Similar articles
- Repurposing Hsp104 to Antagonize Seminal Amyloid and Counter HIV Infection.
Castellano LM, Bart SM, Holmes VM, Weissman D, Shorter J. Castellano LM, et al. Chem Biol. 2015 Aug 20;22(8):1074-86. doi: 10.1016/j.chembiol.2015.07.007. Epub 2015 Aug 6. Chem Biol. 2015. PMID: 26256479 Free PMC article. - The Surprising Role of Amyloid Fibrils in HIV Infection.
Castellano LM, Shorter J. Castellano LM, et al. Biology (Basel). 2012 May 29;1(1):58-80. doi: 10.3390/biology1010058. Biology (Basel). 2012. PMID: 24832047 Free PMC article. - ADS-J1 disaggregates semen-derived amyloid fibrils.
Li J, Yang Z, Liu H, Qiu M, Zhang T, Li W, Li Z, Qi T, Qiu Y, Li L, Zhou X, Liu S, Tan S. Li J, et al. Biochem J. 2019 Mar 29;476(6):1021-1035. doi: 10.1042/BCJ20180886. Biochem J. 2019. PMID: 30877194 - [Semen-derived enhancer of viral infection--a key factor in sexual transmission of HIV].
Duan JM, Qiu JY, Tan SY, Liu SW, Li L. Duan JM, et al. Bing Du Xue Bao. 2012 Jan;28(1):84-8. Bing Du Xue Bao. 2012. PMID: 22416356 Review. Chinese. - Natural Seminal Amyloids as Targets for Development of Synthetic Inhibitors of HIV Transmission.
Sheik DA, Dewhurst S, Yang J. Sheik DA, et al. Acc Chem Res. 2017 Sep 19;50(9):2159-2166. doi: 10.1021/acs.accounts.7b00154. Epub 2017 Aug 15. Acc Chem Res. 2017. PMID: 28809479 Review.
Cited by
- Gallic Acid Is an Antagonist of Semen Amyloid Fibrils That Enhance HIV-1 Infection.
LoRicco JG, Xu CS, Neidleman J, Bergkvist M, Greene WC, Roan NR, Makhatadze GI. LoRicco JG, et al. J Biol Chem. 2016 Jul 1;291(27):14045-14055. doi: 10.1074/jbc.M116.718684. Epub 2016 May 11. J Biol Chem. 2016. PMID: 27226574 Free PMC article. - Semen-derived amyloidogenic peptides-Key players of HIV infection.
Lee YH, Ramamoorthy A. Lee YH, et al. Protein Sci. 2018 Jul;27(7):1151-1165. doi: 10.1002/pro.3395. Epub 2018 Mar 14. Protein Sci. 2018. PMID: 29493036 Free PMC article. Review. - An Overview on the Potential Roles of EGCG in the Treatment of COVID-19 Infection.
Bimonte S, Forte CA, Cuomo M, Esposito G, Cascella M, Cuomo A. Bimonte S, et al. Drug Des Devel Ther. 2021 Oct 28;15:4447-4454. doi: 10.2147/DDDT.S314666. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34737551 Free PMC article. Review. - Epigallocatechin-3-gallate local pre-exposure application prevents SHIV rectal infection of macaques.
Liu JB, Li JL, Zhuang K, Liu H, Wang X, Xiao QH, Li XD, Zhou RH, Zhou L, Ma TC, Zhou W, Liu MQ, Ho WZ. Liu JB, et al. Mucosal Immunol. 2018 Jul;11(4):1230-1238. doi: 10.1038/s41385-018-0025-4. Epub 2018 May 31. Mucosal Immunol. 2018. PMID: 29855550 Free PMC article. - (-)-Epigallocatechin-3-gallate induces interferon-λ2 expression to anti-influenza A virus in human bronchial epithelial cells (BEAS-2B) through p38 MAPK signaling pathway.
Zhu J, Ou L, Zhou Y, Yang Z, Bie M. Zhu J, et al. J Thorac Dis. 2020 Mar;12(3):989-997. doi: 10.21037/jtd.2020.03.20. J Thorac Dis. 2020. PMID: 32274168 Free PMC article.
References
- Arnold F., Schnell J., Zirafi O., Sturzel C., Meier C., Weil T., Standker L., Forssmann W.-G., Roan N. R., Greene W. C. et al. (2012). Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. J. Virol. 86, 1244-1249. 10.1128/JVI.06121-11 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources