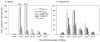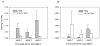Anti-tumor effect of the alphavirus-based virus-like particle vector expressing prostate-specific antigen in a HLA-DR transgenic mouse model of prostate cancer - PubMed (original) (raw)
Anti-tumor effect of the alphavirus-based virus-like particle vector expressing prostate-specific antigen in a HLA-DR transgenic mouse model of prostate cancer
V Riabov et al. Vaccine. 2015.
Abstract
The goal of this study was to determine if an alphavirus-based vaccine encoding human Prostate-Specific Antigen (PSA) could generate an effective anti-tumor immune response in a stringent mouse model of prostate cancer. DR2bxPSA F1 male mice expressing human PSA and HLA-DRB1(*)1501 transgenes were vaccinated with virus-like particle vector encoding PSA (VLPV-PSA) followed by the challenge with Transgenic Adenocarcinoma of Mouse Prostate cells engineered to express PSA (TRAMP-PSA). PSA-specific cellular and humoral immune responses were measured before and after tumor challenge. PSA and CD8 reactivity in the tumors was detected by immunohistochemistry. Tumor growth was compared in vaccinated and control groups. We found that VLPV-PSA could infect mouse dendritic cells in vitro and induce a robust PSA-specific immune response in vivo. A substantial proportion of splenic CD8 T cells (19.6 ± 7.4%) produced IFNγ in response to the immunodominant peptide PSA(65-73). In the blood of vaccinated mice, 18.4 ± 4.1% of CD8 T cells were PSA-specific as determined by the staining with H-2D(b)/PSA(65-73) dextramers. VLPV-PSA vaccination also strongly stimulated production of IgG2a/b anti-PSA antibodies. Tumors in vaccinated mice showed low levels of PSA expression and significant CD8+ T cell infiltration. Tumor growth in VLPV-PSA vaccinated mice was significantly delayed at early time points (p=0.002, Gehan-Breslow test). Our data suggest that TC-83-based VLPV-PSA vaccine can efficiently overcome immune tolerance to PSA, mediate rapid clearance of PSA-expressing tumor cells and delay tumor growth. The VLPV-PSA vaccine will undergo further testing for the immunotherapy of prostate cancer.
Keywords: DR2b mice; PSA; Prostate cancer; TC-83 virus; VLPV; Vaccine.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement.
There are no conflicts of interest among the authors regarding the work.
Figures
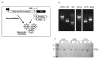
Figure 1. VLPV-PSA vaccine design
A. Schema of the TC-83 vaccine replicon vector containing cloned PSA gene, and a bipartite packaging helper. Indicated are the T7 RNA polymerase promoter (filled arrow), the 26S subgenomic promoter (open arrows), and the location of the PSA gene in the vaccine vector. B. Production of RNA for transfection into CHO cells. RNAs were made by run-off in vitro transcriptions using T7 RNA polymerase in the presence of 5′ cap analog. The order of the samples as follows: wild-type PSA (wtPSA); M1 and M2, RNA and DNA control markers; codon-optimized PSA (coPSA); C276 c-helper and gp23 gp-helper. C. Expression of PSA by Western blot. CHO-K1 cells were transfected with vector RNA encoding PSA variants, culture supernatants were collected at indicated time points. Western blot was carried out using antiserum specific for human PSA. “wt”, “co”, “nc” indicate wtPSA, coPSA, and negative control respectively.
Figure 2. VLPV-PSA vaccine induces dose-dependent PSA-specific immune response in DR2bxPSA F1 mice
Mice were immunized i.m. twice with VLPV-PSA at indicated doses. Cellular immune responses to PSA were measured in the spleens (A) and inguinal LN (B) two weeks after boost immunization using IFNγ ELISPOT assay. Lymphocytes (2×105 per well) were stimulated with either H-2Db restricted peptide PSA65–73 or TRAMP-PSA tumor targets. Irrelevant peptide Neo49–59, parental TRAMP-C1 tumor cells or media (No Ag) were used as negative controls. Target tumor cells were pre-treated with IFNγ and irradiated at 10,000 rad. The data for the pool of 3 mice per group are shown (mean ± SD of triplicates). TNTC: too numerous to count.
Figure 3. Immunogenicity of VLPV-PSA vaccine in DR2bxPSA F1 mice
A. Direct detection of PSA-specific CD8+ T cells in the peripheral circulation. Peripheral blood was stained with H-2Db/PSA65–73 Dxt PE in combination with anti-CD8 FITC and CD62L Alexa Fluor 647 mAbs. Percentages of the Dxt+CD62L− events within CD8+ gate are shown for a representative VLPV-PSA-vaccinated (left panel) or naïve (right panel) mouse. B. Intracellular cytokine staining. Splenocytes were stimulated for 16 hr with H-2Db-restricted peptide PSA65–73 or left untreated (No Ag). Cells stimulated with PMA/Ionomycin were used as positive controls (data not shown). Cells were stained with anti-CD8 PerCP Cy5.5 mAb followed by intracellular staining with anti-IFNγ PE mAb. Percentages of events within CD8+ gate are shown for individual mice from a representative experiment. C. Anti-PSA antibody responses. Heparinized plasma was analyzed for the presence of anti-PSA Abs by ELISA. Anti-PSA ab titers are shown for IgG1, IgG2a, and IgG2b sub-isotypes. Each symbol represents a titer for an individual mouse (n=12). Horizontal lines are geometric means. For the titers above assay upper limit of quantitation values equal to 21,870 were designated.
Figure 4. The kinetics of PSA expression and CD8 T cell infiltration in TRAMP-PSA tumors
Mice were immunized with VLPV-PSA followed by the inoculation with TRAMP-PSA tumor cells impregnated in Matrigel. Tumor implants were harvested 1, 2 or 3 weeks later (n=3 per treatment group per time point). Cryosections were stained using either goat anti-human PSA (A) or rat anti-mouse CD8 (B) Abs. Images were taken using Zeiss Axioskop microscope with 2.5X objective using AxioVision 3.0 software. Images were scanned using Aperio ImageScope software and Positive Pixel Count Algorithm. Data are expressed as average intensity of staining (positivity) ± SD. Representative IHC images for individual mice are shown in Supplemental Figure 2 (PSA) and Supplemental Figure 3 (CD8). The global effect of the vaccination analyzed by the ANOVA F test was statistically significant for the PSA staining intensity (A) (p=0.0007), although pairwise comparisons between vaccinated and non-vaccinated groups at each time point were only marginally significant (0.05<p<0.1, Satterthwaite t-test). The global effect of the vaccination for the CD8 staining intensity (B) was not significant (p=0.347). Within the treatment groups, the effect of time was also non-significant (ANOVA F test).
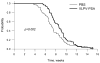
Figure 5. Vaccination with VLPV-PSA delays tumor growth at early time-points
Mice were immunized with VLPV-PSA vaccine twice with 4 weeks interval between primary and secondary immunizations. Four weeks after secondary immunization mice were s.c. injected with TRAMP-PSA tumor cells. Tumor growth was monitored weekly for up to 15 weeks. Combined results from 4 independent experiments with identical trend are shown (n=62 for the control group, n=57 for the vaccine group). Time-to-event analysis (tumor base of 100 mm2) was performed by the Gehan-Breslow test (p value is shown on the graph). The results of the individual experiments are shown in Table 1.
Figure 6. PSA-specific CD8 T cell response after TRAMP-PSA tumor inoculation
Mice were immunized with VLPV-PSA followed by the s.c. injection with TRAMP-PSA tumor cells. A. The kinetics of PSA-specific CD8 T cell response in the peripheral blood. Heparinized blood was collected at indicated time points after tumor inoculation and stained with H-2Db/PSA65–73 Dxt PE, anti-CD8 FITC, and CD62L Alexa Fluor 647 mAbs. Percentages of the Dxt+CD62L− events within CD8+ gate are shown. The difference between week 1 and week 2 was statistically significant (ANOVA F test, p=0.04). The data are averages ± SD (n=3 per group). B. IFNγ ELISPOT assay. Splenocytes from individual mice (2×105 per well, n=3) or DLN pooled from the same mice (4×105 per well) were plated in triplicates and stimulated with either H-2Db restricted peptide PSA65–73 or TRAMP-PSA tumor targets. Irrelevant peptide Neo49–59, parental TRAMP-C1 tumor cells or media (No Ag) were used as negative controls. Frequencies of the IFNγ-producing cells per 1×106 were calculated as described in the “Materials and Methods” section. C. Intracellular cytokine staining. Splenocytes were stimulated as described in the legend to Figure 2B. Cells were stained with anti-CD8 PerCP Cy5.5 mAb followed by intracellular staining with anti-IFNγ PE and anti-TNFα FITC mAbs. The dot plots for the representative vaccinated and control mouse are shown. Numbers are percentages of events in each quadrant within CD8+ gated population.
Similar articles
- A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer.
Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA. Klyushnenkova EN, et al. J Immunother. 2012 Jun;35(5):390-9. doi: 10.1097/CJI.0b013e3182585d50. J Immunother. 2012. PMID: 22576344 Free PMC article. - Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice.
Karan D, Dubey S, Van Veldhuizen P, Holzbeierlein JM, Tawfik O, Thrasher JB. Karan D, et al. Immunotherapy. 2011 Jun;3(6):735-46. doi: 10.2217/imt.11.59. Immunotherapy. 2011. PMID: 21668311 - Breaking immune tolerance by targeting CD25+ regulatory T cells is essential for the anti-tumor effect of the CTLA-4 blockade in an HLA-DR transgenic mouse model of prostate cancer.
Klyushnenkova EN, Riabov VB, Kouiavskaia DV, Wietsma A, Zhan M, Alexander RB. Klyushnenkova EN, et al. Prostate. 2014 Oct;74(14):1423-32. doi: 10.1002/pros.22858. Epub 2014 Aug 11. Prostate. 2014. PMID: 25111463 - CpG oligonucleotide as an adjuvant for the treatment of prostate cancer.
Lubaroff DM, Karan D. Lubaroff DM, et al. Adv Drug Deliv Rev. 2009 Mar 28;61(3):268-74. doi: 10.1016/j.addr.2008.12.005. Epub 2009 Jan 7. Adv Drug Deliv Rev. 2009. PMID: 19166887 Review. - Prostate cancer vaccines: current status and future potential.
Doehn C, Böhmer T, Kausch I, Sommerauer M, Jocham D. Doehn C, et al. BioDrugs. 2008;22(2):71-84. doi: 10.2165/00063030-200822020-00001. BioDrugs. 2008. PMID: 18345705 Review.
Cited by
- Virus-like particle vaccines: immunology and formulation for clinical translation.
Donaldson B, Lateef Z, Walker GF, Young SL, Ward VK. Donaldson B, et al. Expert Rev Vaccines. 2018 Sep;17(9):833-849. doi: 10.1080/14760584.2018.1516552. Epub 2018 Sep 19. Expert Rev Vaccines. 2018. PMID: 30173619 Free PMC article. Review. - DNA-launched live-attenuated vaccines for biodefense applications.
Pushko P, Lukashevich IS, Weaver SC, Tretyakova I. Pushko P, et al. Expert Rev Vaccines. 2016 Sep;15(9):1223-34. doi: 10.1080/14760584.2016.1175943. Epub 2016 Apr 25. Expert Rev Vaccines. 2016. PMID: 27055100 Free PMC article. Review. - Single-Dose Immunogenic DNA Vaccines Coding for Live-Attenuated Alpha- and Flaviviruses.
Pushko P, Lukashevich IS, Johnson DM, Tretyakova I. Pushko P, et al. Viruses. 2024 Mar 10;16(3):428. doi: 10.3390/v16030428. Viruses. 2024. PMID: 38543793 Free PMC article. Review. - Replicon RNA Viral Vectors as Vaccines.
Lundstrom K. Lundstrom K. Vaccines (Basel). 2016 Nov 7;4(4):39. doi: 10.3390/vaccines4040039. Vaccines (Basel). 2016. PMID: 27827980 Free PMC article. Review. - Emerging Concepts and Technologies in Vaccine Development.
Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Brisse M, et al. Front Immunol. 2020 Sep 30;11:583077. doi: 10.3389/fimmu.2020.583077. eCollection 2020. Front Immunol. 2020. PMID: 33101309 Free PMC article. Review.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar;61(2):69–90. - PubMed
- Karan D, Holzbeierlein JM, Van VP, Thrasher JB. Cancer immunotherapy: a paradigm shift for prostate cancer treatment. Nat Rev Urol. 2012 Jul;9(7):376–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous