Assessing Bladder Cancer Risk in Type 2 Diabetes Clinical Trials: the Dapagliflozin Drug Development Program as a 'Case Study' - PubMed (original) (raw)
Assessing Bladder Cancer Risk in Type 2 Diabetes Clinical Trials: the Dapagliflozin Drug Development Program as a 'Case Study'
Agata Ptaszynska et al. Diabetes Ther. 2015 Sep.
Abstract
Introduction: Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, decreases plasma glucose levels by suppressing renal glucose reabsorption and increasing urinary glucose excretion. Previously published pre-clinical data suggest that dapagliflozin lacks carcinogenic potential. This article reviews data on bladder cancer with dapagliflozin to illustrate the challenges in assessing bladder cancer in drug development programs in patients with type 2 diabetes mellitus (T2DM).
Methods: Clinical cases of bladder cancer were analyzed in a pooled population of >9000 patients in 21 phase 2b/3 dapagliflozin clinical trials of up to 208 weeks' duration.
Results: In the 21-study pool, demographic and baseline characteristics were generally consistent between dapagliflozin and comparator groups. The overall incidence of malignancies was also balanced between the treatment groups, with an incidence rate ratio (IRR) of 1.035 [95% confidence interval (CI): 0.724, 1.481]. Nine of 5936 dapagliflozin-treated patients and 1 of 3403 comparator-treated patients reported bladder cancer, with an IRR of 5.168 (95% CI: 0.677, 233.55). All of these patients had clinical attributes typical of bladder cancer in the general population (≥60-year-old males; 8 of the 10 patients were current/former smokers). All cases of bladder cancer were reported within 2 years of starting study treatment. There was an absence of detailed workup of hematuria prior to randomization, and no hematuria workup data were collected proactively in the dapagliflozin trials, which is typical of clinical practice. Failure to exclude bladder cancer prior to randomization increases the chance of recruiting patients with pre-existing bladder cancer in clinical trials and may delay the final diagnosis. Of the nine dapagliflozin-treated patients with bladder cancer, eight had microscopic hematuria prior to start of treatment or within 6 months of initiating study treatment.
Conclusion: The assessment of bladder cancer data illustrates the challenges of characterizing cancer risk in T2DM drug development programs. The totality of evidence to date does not suggest a causal relationship between dapagliflozin and bladder cancer.
Funding: AstraZeneca.
Keywords: Bladder cancer; Dapagliflozin; Hematuria; SGLT2 inhibitor; Type 2 diabetes mellitus.
Figures
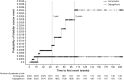
Fig. 1
Incident rate ratio of malignant and unspecified tumors by organ category from the dapagliflozin clinical trials. N is the number of treated patients. Only trials with at least one selected event contributed to the analysis. Confidence intervals are based on an exact method. Includes serious and non-serious adverse events on or after the first date/time of double-blind treatment and on or prior to the last day of short-term plus long-term treatment +30 days (or up to follow-up visit whichever was first) or +4 days
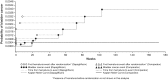
Fig. 2
Kaplan–Meier plot of time to first event of bladder cancer. Includes data after rescue. Week is not scheduled week visit, but actual days from the first dose of double-blind study medication divided by 7. The number of patients at risk is the number of patients at risk in the beginning of the period
Fig. 3
Relationship between first hematuria event after randomization and time to first bladder cancer event
Similar articles
- Effect of the SGLT2 Inhibitor Dapagliflozin on Potassium Levels in Patients with Type 2 Diabetes Mellitus: A Pooled Analysis.
Yavin Y, Mansfield TA, Ptaszynska A, Johnsson K, Parikh S, Johnsson E. Yavin Y, et al. Diabetes Ther. 2016 Mar;7(1):125-37. doi: 10.1007/s13300-015-0150-y. Epub 2016 Jan 12. Diabetes Ther. 2016. PMID: 26758563 Free PMC article. - Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes.
Hinnen D. Hinnen D. Ther Adv Endocrinol Metab. 2015 Jun;6(3):92-102. doi: 10.1177/2042018815575273. Ther Adv Endocrinol Metab. 2015. PMID: 26137213 Free PMC article. Review. - Dapagliflozin in patients with type 2 diabetes mellitus.
Filippatos TD, Liberopoulos EN, Elisaf MS. Filippatos TD, et al. Ther Adv Endocrinol Metab. 2015 Feb;6(1):29-41. doi: 10.1177/2042018814558243. Ther Adv Endocrinol Metab. 2015. PMID: 25678954 Free PMC article. Review. - Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus.
Reilly TP, Graziano MJ, Janovitz EB, Dorr TE, Fairchild C, Lee F, Chen J, Wong T, Whaley JM, Tirmenstein M. Reilly TP, et al. Diabetes Ther. 2014 Jun;5(1):73-96. doi: 10.1007/s13300-014-0053-3. Epub 2014 Jan 29. Diabetes Ther. 2014. PMID: 24474422 Free PMC article. - Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents.
Vivian EM. Vivian EM. Drugs Context. 2014 Dec 19;3:212264. doi: 10.7573/dic.212264. eCollection 2014. Drugs Context. 2014. PMID: 25598831 Free PMC article. Review.
Cited by
- Associations between Diabetes Mellitus and Selected Cancers.
Pliszka M, Szablewski L. Pliszka M, et al. Int J Mol Sci. 2024 Jul 8;25(13):7476. doi: 10.3390/ijms25137476. Int J Mol Sci. 2024. PMID: 39000583 Free PMC article. Review. - Cancer and Cardiovascular Disease: The Conjoined Twins.
Zmaili M, Alzubi J, Alkhayyat M, Albakri A, Alkhalaileh F, Longinow J, Moudgil R. Zmaili M, et al. Cancers (Basel). 2024 Apr 9;16(8):1450. doi: 10.3390/cancers16081450. Cancers (Basel). 2024. PMID: 38672532 Free PMC article. Review. - Cancer biology in diabetes update: Focusing on antidiabetic drugs.
Kawakita E, Kanasaki K. Kawakita E, et al. J Diabetes Investig. 2024 May;15(5):525-540. doi: 10.1111/jdi.14152. Epub 2024 Mar 8. J Diabetes Investig. 2024. PMID: 38456597 Free PMC article. Review. - Association between diabetes and cancer. Current mechanistic insights into the association and future challenges.
Rojas A, Schneider I, Lindner C, Gonzalez I, Morales MA. Rojas A, et al. Mol Cell Biochem. 2023 Aug;478(8):1743-1758. doi: 10.1007/s11010-022-04630-x. Epub 2022 Dec 24. Mol Cell Biochem. 2023. PMID: 36565361 Review. - SGLT-2 Inhibitors in Cancer Treatment-Mechanisms of Action and Emerging New Perspectives.
Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T, Ćwiertnia M, Hajduga MB. Dutka M, et al. Cancers (Basel). 2022 Nov 25;14(23):5811. doi: 10.3390/cancers14235811. Cancers (Basel). 2022. PMID: 36497303 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources