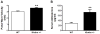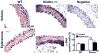Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy - PubMed (original) (raw)
Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy
Kai Chen et al. Hypertension. 2015 Nov.
Abstract
The prevalence of arterial stiffness increases with age, whereas the level of the aging-suppressor protein klotho decreases with age. The objective of this study is to assess whether haplodeficiency of klotho gene causes arterial stiffness and to investigate the underlying mechanism. Pulse wave velocity, a direct measure of arterial stiffness, was increased significantly in klotho heterozygous (klotho(+/-)) mice versus their age-matched wild-type (WT) littermates, suggesting that haplodeficiency of klotho causes arterial stiffening. Notably, plasma aldosterone levels were elevated significantly in klotho(+/-) mice. Treatment with eplerenone (6 mg/kg per day IP), an aldosterone receptor blocker, abolished klotho deficiency-induced arterial stiffening in klotho(+/-) mice. Klotho deficiency was associated with increased collagen and decreased elastin contents in the media of aortas. In addition, arterial matrix metalloproteinase-2, matrix metalloproteinase-9, and transforming growth factor-β1 expression and myofibroblast differentiation were increased in klotho(+/-) mice. These klotho deficiency-related changes can be blocked by eplerenone. Protein expression of scleraxis, a transcription factor for collagen synthesis, and LC3-II/LC3-I, an index of autophagy, were upregulated in aortas of klotho(+/-) mice, which can be abolished by eplerenone. In cultured mouse aortic smooth muscle cells, aldosterone increased collagen-1 expression that can be completely eliminated by small interfering RNA knockdown of scleraxis. Interestingly, aldosterone decreased elastin levels in smooth muscle cells, which can be abolished by small interfering RNA knockdown of Beclin-1, an autophagy-related gene. In conclusion, this study demonstrated for the first time that klotho deficiency-induced arterial stiffening may involve aldosterone-mediated upregulation of scleraxis and induction of autophagy, which led to increased collagen-1 expression and decreased elastin levels, respectively.
Keywords: Becn1 protein, mouse; Scx protein, mouse; autophagy; collagen; elastin; myofibroblasts; vascular stiffness.
© 2015 American Heart Association, Inc.
Figures
Figure 1
Haplodeficiency of Klotho gene (klotho+/−) increased arterial pulse wave velocity (PWV) and serum aldosterone levels. (A) PWV was measured in klotho+/− and age-mated WT mice by 10-MHz Doppler probes (n=14). (B) Serum aldosterone levels were measured by ELISA (n=6). Data are expressed as mean±SE and analyzed by a one-way ANOVA. **p<0.01 vs. WT group.
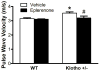
Figure 2
Blockade of aldosterone receptors abolished the increase of PWV in klotho+/− mice. PWV was measured after treatment with eplerenone for 3 weeks. Data are expressed as mean±SE and analyzed by two-way ANOVA. n=7. *p<0.05 vs. WT group; #p<0.05 vs. klotho+/−-vehicle group.
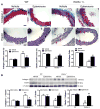
Figure 3
Haplodeficiency of klotho gene increased collagen expression but decreased elastin levels in aortas which can be abolished by blockade of aldosterone receptors. (A) Immunohistochemical analysis of collagen-1 (blue) and elastin (brown). (B) Western blot analysis of collagen-1 and elastin. Data are expressed as mean±SE and analyzed by a two-way ANOVA. n=5. *p<0.05, **p<0.01 vs. WT group; #p<0.05, ##p<0.01 vs. klotho+/−-vehicle group. Scale bar = 20 μm.
Figure 4
Haplodeficiency of klotho gene increased arterial MMP2, MMP9 and TGFβ1 expression which can be eliminated by blockade of aldosterone receptors. (A) Immunohistochemical staining results of MMP2 and MMP9. (B) Western blot analysis of MMP2 and MMP9 expression. (C) Western blot analysis of TGFβ1 expression. Data are expressed as mean±SE and analyzed by two-way ANOVA. n=5, *p<0.05, **p<0.01 vs WT group; #p<0.05, ##p<0.01 vs klotho+/−-vehicle group. Scale bar = 20 μm
Figure 5
Klotho+/− increased aortic myofibroblasts differentiation which can be abolished by blockade of aldosterone receptors. Myofibroblast differentiation was evaluated by α-SMA positive cells using immunohistochemical staining. The semi-quantitative data are expressed as mean±SE and analyzed by two-way ANOVA. n=5, **p<0.01 vs WT group; #p<0.05 vs klotho+/−-vehicle group. Scale bar = 20 μm.
Similar articles
- Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity.
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Gao D, et al. Hypertension. 2016 Nov;68(5):1191-1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. Epub 2016 Sep 12. Hypertension. 2016. PMID: 27620389 Free PMC article. - Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension.
Chen K, Sun Z. Chen K, et al. J Mol Med (Berl). 2019 Nov;97(11):1615-1625. doi: 10.1007/s00109-019-01841-6. Epub 2019 Oct 19. J Mol Med (Berl). 2019. PMID: 31630227 Free PMC article. - Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase.
Lin Y, Chen J, Sun Z. Lin Y, et al. Hypertension. 2016 Mar;67(3):564-73. doi: 10.1161/HYPERTENSIONAHA.115.06825. Epub 2016 Jan 18. Hypertension. 2016. PMID: 26781278 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - Is It Good to Have a Stiff Aorta with Aging? Causes and Consequences.
Pierce GL, Coutinho TA, DuBose LE, Donato AJ. Pierce GL, et al. Physiology (Bethesda). 2022 May 1;37(3):154-173. doi: 10.1152/physiol.00035.2021. Epub 2021 Nov 15. Physiology (Bethesda). 2022. PMID: 34779281 Free PMC article. Review.
Cited by
- Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity.
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Gao D, et al. Hypertension. 2016 Nov;68(5):1191-1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. Epub 2016 Sep 12. Hypertension. 2016. PMID: 27620389 Free PMC article. - Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease.
Donato AJ, Machin DR, Lesniewski LA. Donato AJ, et al. Circ Res. 2018 Sep 14;123(7):825-848. doi: 10.1161/CIRCRESAHA.118.312563. Circ Res. 2018. PMID: 30355078 Free PMC article. Review. - Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging.
El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. El Assar M, et al. Int J Mol Sci. 2022 Aug 5;23(15):8713. doi: 10.3390/ijms23158713. Int J Mol Sci. 2022. PMID: 35955849 Free PMC article. Review. - The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin.
Aroor AR, Manrique-Acevedo C, DeMarco VG. Aroor AR, et al. Cardiovasc Diabetol. 2018 Apr 18;17(1):59. doi: 10.1186/s12933-018-0704-1. Cardiovasc Diabetol. 2018. PMID: 29669555 Free PMC article. Review. - Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension.
Brown IAM, Diederich L, Good ME, DeLalio LJ, Murphy SA, Cortese-Krott MM, Hall JL, Le TH, Isakson BE. Brown IAM, et al. Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):1969-1985. doi: 10.1161/ATVBAHA.118.311229. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354262 Free PMC article. Review.
References
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK093403/DK/NIDDK NIH HHS/United States
- HL105302/HL/NHLBI NIH HHS/United States
- AG049780/AG/NIA NIH HHS/United States
- R01 AG049780/AG/NIA NIH HHS/United States
- R01 HL102074/HL/NHLBI NIH HHS/United States
- HL102074/HL/NHLBI NIH HHS/United States
- P20 GM104934/GM/NIGMS NIH HHS/United States
- R01 HL118558/HL/NHLBI NIH HHS/United States
- R01 HL105302/HL/NHLBI NIH HHS/United States
- HL122166/HL/NHLBI NIH HHS/United States
- R01 HL122166/HL/NHLBI NIH HHS/United States
- R01 DK093403/DK/NIDDK NIH HHS/United States
- HL116863/HL/NHLBI NIH HHS/United States
- R01 HL116863/HL/NHLBI NIH HHS/United States
- 9P20GM104934-06/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases