Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages - PubMed (original) (raw)
Review
Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages
Stefano Ugel et al. J Clin Invest. 2015 Sep.
Abstract
The generation of an inflammatory environment is favorable and often decisive for the growth of both primary tumors and metastases. Tumor cells either express membrane molecules or release tumor-derived soluble factors able to alter myelopoiesis. Tumor-reprogrammed myeloid cells not only create a tolerogenic environment by blocking T cell functions and proliferation, but also directly drive tumor growth by promoting cancer stemness, angiogenesis, stroma deposition, epithelial-to-mesenchymal transition, and metastasis formation. In this Review, we discuss the interplay between immunosuppressive and protumoral myeloid cells and detail their immune-regulatory mechanisms, the molecular pathways involved in their differentiation, as well as their potential role as prognostic and diagnostic biomarkers and prospective targets for innovative approaches to treat tumor-bearing hosts.
Figures
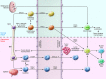
Figure 3. TAM- and MDSC-dependent mechanisms driving tumor progression.
TAMs and MDSCs sustain tumor growth, progression, and dissemination by promoting immune dysfunction (green slices) but also by nonimmune-related mechanisms (yellow slices). (A) TAMs alter immune responses in tumor-bearing hosts by four main mechanisms: 1) inhibition of T cell activation; 2) inhibition of T cell viability; 3) promotion of Treg induction and recruitment; and 4) consumption of metabolites essential for T cell fitness. TAMs promote tumor angiogenesis and vasculogenesis by the release of VEGF and WNT7β, which favor the generation of new blood vessels and sustain metastasis. Finally, TAMs maintain the cancer cell reservoir by secreting IL-6 and TNF-α and produce MFG-E8 to protect CSCs from chemotherapy. (B) MDSCs inhibit the immune response in tumor-bearing mice by four processes: 1) MDSCs drive the differentiation of immune cells toward regulatory cells; 2) MDSCs interfere with T cell migration and viability; 3) MDSCs alter T cell fitness by turning on intracellular ARG1, NOS2, and NOX2 expression to produce NO, ROS, and RNS (ONOO–, O2–, H2O2); and 4) MDSCs deplete essential metabolites for T lymphocyte fitness. MDSCs can also promote tumor angiogenesis and vasculogenesis via VEGF and MMP9 secretion. MDSCs produce elevated levels of TGF-β and HGF in primary tumors, inducing EMT, and secrete versican in the metastatic niche, promoting MET. Finally, MDSCs maintain tumor cell stemness by both IL-1RA production and by inducing the upregulation of miR-101 in cancer stem cells. cGMP, cyclic GMP; βcat, β-catenin; N, nitrosylated/nitrated; Tcf, HNF1 homeobox A.
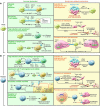
Figure 2. MDSC and TAM development in tumor-bearing mice.
Under steady-state conditions, resident macrophages may originate from either embryonic tissues or inflammatory monocytes. Resident macrophages are programmed by local factors, and molecular switches support their differentiation. Circulating monocytes can be divided into two subsets: patrolling monocytes (Ly6CloCX3CR1hi) and inflammatory monocytes (Ly6ChiCD11b+CD11c–MHCII–VCAM1–CCR2+), originating from macrophage and DC precursors (MDPs) in BM. Inflammatory monocytes migrate from blood to tissue under the guidance of CCL2/CCR2 chemokine signaling. Tumor cells secrete several factors that modify physiological myelopoiesis, promoting MDP differentiation into PMN-MDSCs (CD11b+Ly6G+) and MO-MDSCs (CD11b+Ly6ChiCCR2+CD115+F4/80lo). MO-MDSCs also originate from the spleen under conditions of emergency and reactive myelopoiesis. MO-MDSCs and inflammatory monocytes migrate to tumor tissues via CCL2/CCR2 and CSF1 signaling and differentiate into TAMs (Ly6C–CD11b+/loCD68+CD1d+MHCIIhi/loF4/80+VCAM1+) in the presence of specific signals released by tumor cells within the local environment. However, the TAM phenotypic profile depends on cancer histology and stage, which might influence marker distribution. TAMs also proliferate locally, with different rates in various tumors. Furthermore, TAMs are inherently plastic, with an activation state falling along a continuum between the two extremes of M1- and M2-like phenotypes. Rb, retinoblastoma.
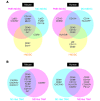
Figure 1. Common phenotypic markers of MDSCs and TAMs.
Several phenotypic markers of mouse and human MDSCs (A) and TAMs (B) have been identified (+ indicates expression, while – indicates lack of expression) and used to define specific cell subgroups, such as PMN-MDSCs, MO-MDSCs, and immature MDSCs (I-MDSCs), as well as M1-like and M2-like TAMs, by both cytofluorimetric and immunohistochemical analyses.
Similar articles
- Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas.
Hamilton MJ, Bosiljcic M, Lepard NE, Halvorsen EC, Ho VW, Banáth JP, Krystal G, Bennewith KL. Hamilton MJ, et al. J Immunol. 2014 Jan 1;192(1):512-22. doi: 10.4049/jimmunol.1300096. Epub 2013 Nov 27. J Immunol. 2014. PMID: 24285836 - Pro-Tumoral Inflammatory Myeloid Cells as Emerging Therapeutic Targets.
Szebeni GJ, Vizler C, Nagy LI, Kitajka K, Puskas LG. Szebeni GJ, et al. Int J Mol Sci. 2016 Nov 23;17(11):1958. doi: 10.3390/ijms17111958. Int J Mol Sci. 2016. PMID: 27886105 Free PMC article. Review. - Altered macrophage differentiation and immune dysfunction in tumor development.
Sica A, Bronte V. Sica A, et al. J Clin Invest. 2007 May;117(5):1155-66. doi: 10.1172/JCI31422. J Clin Invest. 2007. PMID: 17476345 Free PMC article. Review. - Tumor-Induced Myeloid-Derived Suppressor Cells.
De Sanctis F, Bronte V, Ugel S. De Sanctis F, et al. Microbiol Spectr. 2016 Jun;4(3). doi: 10.1128/microbiolspec.MCHD-0016-2015. Microbiol Spectr. 2016. PMID: 27337449 Review. - Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected.
Marvel D, Gabrilovich DI. Marvel D, et al. J Clin Invest. 2015 Sep;125(9):3356-64. doi: 10.1172/JCI80005. Epub 2015 Jul 13. J Clin Invest. 2015. PMID: 26168215 Free PMC article. Review.
Cited by
- Tumor microenvironment in pancreatic ductal adenocarcinoma: Implications in immunotherapy.
Smith C, Zheng W, Dong J, Wang Y, Lai J, Liu X, Yin F. Smith C, et al. World J Gastroenterol. 2022 Jul 21;28(27):3297-3313. doi: 10.3748/wjg.v28.i27.3297. World J Gastroenterol. 2022. PMID: 36158269 Free PMC article. Review. - The importance of cancer-associated fibroblasts in targeted therapies and drug resistance in breast cancer.
Zheng J, Hao H. Zheng J, et al. Front Oncol. 2024 Jan 4;13:1333839. doi: 10.3389/fonc.2023.1333839. eCollection 2023. Front Oncol. 2024. PMID: 38273859 Free PMC article. Review. - CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies.
Huang J, Huang X, Huang J. Huang J, et al. Front Immunol. 2022 Sep 28;13:1019115. doi: 10.3389/fimmu.2022.1019115. eCollection 2022. Front Immunol. 2022. PMID: 36248810 Free PMC article. Review. - Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells.
Fan Q, Zuo J, Tian H, Huang C, Nice EC, Shi Z, Kong Q. Fan Q, et al. J Exp Clin Cancer Res. 2022 May 3;41(1):162. doi: 10.1186/s13046-022-02372-8. J Exp Clin Cancer Res. 2022. PMID: 35501823 Free PMC article. - Environmental exposure and the role of AhR in the tumor microenvironment of breast cancer.
Sweeney C, Lazennec G, Vogel CFA. Sweeney C, et al. Front Pharmacol. 2022 Dec 15;13:1095289. doi: 10.3389/fphar.2022.1095289. eCollection 2022. Front Pharmacol. 2022. PMID: 36588678 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources