How to operate a nuclear pore complex by Kap-centric control - PubMed (original) (raw)
Review
How to operate a nuclear pore complex by Kap-centric control
Roderick Y H Lim et al. Nucleus. 2015.
Abstract
Nuclear pore complexes (NPCs) mediate molecular transport between the nucleus and cytoplasm in eukaryotic cells. Tethered within each NPC lie numerous intrinsically disordered proteins known as FG nucleoporins (FG Nups) that are central to this process. Over two decades of investigation has converged on a view that a barrier mechanism consisting of FG Nups rejects non-specific macromolecules while promoting the speed and selectivity of karyopherin (Kaps) receptors (and their cargoes). Yet, the number of NPCs in the cell is exceedingly small compared to the number of Kaps, so that in fact there is a high likelihood the pores are always populated by Kaps. Here, we contemplate a view where Kaps actively participate in regulating the selectivity and speed of transport through NPCs. This so-called "Kap-centric" control of the NPC accounts for Kaps as essential barrier reinforcements that play a prerequisite role in facilitating fast transport kinetics. Importantly, Kap-centric control reconciles both mechanistic and kinetic requirements of the NPC, and in so doing potentially resolves incoherent aspects of FG-centric models. On this basis, we surmise that Kaps prime the NPC for nucleocytoplasmic transport by fine-tuning the NPC microenvironment according to the functional needs of the cell.
Keywords: FG Nucleoporin; Karyopherin; Nuclear pore complex; Nucleocytoplasmic transport.
Figures
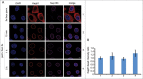
Figure 1.
The NPC-bound fraction of endogenous Kapβ1 is stable and long-lived. (A) Row 1: HeLa cells were fixed and digitonin-permeabilized as described in ref. 56. As a control, anti-Kapβ1 (Abcam) staining reveals the steady state distribution of endogenous Kapβ1, a fraction of which is localized at the nuclear envelope. This further co-localizes with anti-Nup153 (Sigma) staining, thereby signifying the presence of Kapβ1 at the NPCs. Row 2 to 4: To test for the retention of the NPC-bound pool of endogenous Kapβ1, cells were first digitonin-permeabilized then incubated in PBS buffer for 15 mins, 1 hr and 5 hr respectively, followed by fixation. The persistence of anti-Kapβ1 staining at the nuclear envelope up to 5h incubation indicates that the NPC-bound fraction of Kapβ1 is very stable and long-lived. Scale bar, 5 μm. (B) The immunofluorescent staining of Kapβ1 localized at the nuclear envelope was calibrated against InSpeck™ Red calibration beads (Life Technologies) to exclude intensity variations between measurements. The data shown corresponds to Rows 1 to 4 in (A). Final intensity ratios are normalized to the control sample (Row 1) to facilitate comparisons between experiments. Student's t-test analysis (p>0.05) shows a neglectable difference among these experimental outcomes. All images were obtained using a point scanning laser confocal microscope (LSM700, Zeiss AG).
Figure 2.
Hypothetical translocation scenarios in the NPC. Left to right: Small passive molecules that diffuse into the NPC exhibit long 3D search trajectories (and therefore long dwell times) due to a lack of FG-Nup binding. The dirty velcro effect reduces the travel distance of Kaps by promoting 2D diffusion along the luminal surface of the translocation corridor. This reduction of dimensionality leads to shorter dwell times and rapid translocation rates. Large non-specific molecules experience hindered diffusion and have a lower probability to enter the pore. Increased pore confinement and the dirty velcro effect expedite the translocation of large cargoes with multiple Kaps due to reduced search trajectories that stem from 1D diffusion. Note: For clarity of illustration, other diffusing entities have been omitted from each drawing. However, overlaying these scenarios does reveal how different spatiotemporal routes might co-exist in the pore. C = cytoplasm, N = nucleus.
Comment on
- doi: 10.1016/j.bpj.2014.12.041
- doi: 10.1038/nnano.2014.103
- doi: 10.1016/j.bpj.2014.02.021
- doi: 10.1073/pnas.1208440109
Similar articles
- Karyopherins regulate nuclear pore complex barrier and transport function.
Kapinos LE, Huang B, Rencurel C, Lim RYH. Kapinos LE, et al. J Cell Biol. 2017 Nov 6;216(11):3609-3624. doi: 10.1083/jcb.201702092. Epub 2017 Sep 1. J Cell Biol. 2017. PMID: 28864541 Free PMC article. - Karyopherin-centric control of nuclear pores based on molecular occupancy and kinetic analysis of multivalent binding with FG nucleoporins.
Kapinos LE, Schoch RL, Wagner RS, Schleicher KD, Lim RY. Kapinos LE, et al. Biophys J. 2014 Apr 15;106(8):1751-62. doi: 10.1016/j.bpj.2014.02.021. Biophys J. 2014. PMID: 24739174 Free PMC article. - Deciphering networks of protein interactions at the nuclear pore complex.
Allen NP, Patel SS, Huang L, Chalkley RJ, Burlingame A, Lutzmann M, Hurt EC, Rexach M. Allen NP, et al. Mol Cell Proteomics. 2002 Dec;1(12):930-46. doi: 10.1074/mcp.t200012-mcp200. Mol Cell Proteomics. 2002. PMID: 12543930 - Biomechanics of the transport barrier in the nuclear pore complex.
Stanley GJ, Fassati A, Hoogenboom BW. Stanley GJ, et al. Semin Cell Dev Biol. 2017 Aug;68:42-51. doi: 10.1016/j.semcdb.2017.05.007. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506890 Review. - The selective permeability barrier in the nuclear pore complex.
Li C, Goryaynov A, Yang W. Li C, et al. Nucleus. 2016 Sep 2;7(5):430-446. doi: 10.1080/19491034.2016.1238997. Epub 2016 Sep 27. Nucleus. 2016. PMID: 27673359 Free PMC article. Review.
Cited by
- Crowding-induced phase separation of nuclear transport receptors in FG nucleoporin assemblies.
Davis LK, Ford IJ, Hoogenboom BW. Davis LK, et al. Elife. 2022 Jan 31;11:e72627. doi: 10.7554/eLife.72627. Elife. 2022. PMID: 35098921 Free PMC article. - Karyopherins regulate nuclear pore complex barrier and transport function.
Kapinos LE, Huang B, Rencurel C, Lim RYH. Kapinos LE, et al. J Cell Biol. 2017 Nov 6;216(11):3609-3624. doi: 10.1083/jcb.201702092. Epub 2017 Sep 1. J Cell Biol. 2017. PMID: 28864541 Free PMC article. - The Structure of the Nuclear Pore Complex (An Update).
Lin DH, Hoelz A. Lin DH, et al. Annu Rev Biochem. 2019 Jun 20;88:725-783. doi: 10.1146/annurev-biochem-062917-011901. Epub 2019 Mar 18. Annu Rev Biochem. 2019. PMID: 30883195 Free PMC article. Review. - NUP214 in Leukemia: It's More than Transport.
Mendes A, Fahrenkrog B. Mendes A, et al. Cells. 2019 Jan 21;8(1):76. doi: 10.3390/cells8010076. Cells. 2019. PMID: 30669574 Free PMC article. Review. - Physics of the Nuclear Pore Complex: Theory, Modeling and Experiment.
Hoogenboom BW, Hough LE, Lemke EA, Lim RYH, Onck PR, Zilman A. Hoogenboom BW, et al. Phys Rep. 2021 Jul 25;921:1-53. doi: 10.1016/j.physrep.2021.03.003. Epub 2021 Mar 24. Phys Rep. 2021. PMID: 35892075 Free PMC article.
References
- Wente SR, Rout MP. The Nuclear Pore Complex and Nuclear Transport. Cold Spring Harb Perspect Biol 2010; 2:a000562; http://dx.doi.org/10.1101/cshperspect.a000562 - DOI - PMC - PubMed
- Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, Baumeister W, Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science 2004; 306:1387-90; http://dx.doi.org/10.1126/science.1104808 - DOI - PubMed
- Keminer O, Peters R. Permeability of single nuclear pores. Biophys J 1999; 77:217-28; http://dx.doi.org/10.1016/S0006-3495(99)76883-9 - DOI - PMC - PubMed
- Gorlich D, Vogel F, Mills AD, Hartmann E, Laskey RA. Distinct functions for the 2 importin subunits in nuclear-protein import. Nature 1995; 377:246-8; http://dx.doi.org/10.1038/377246a0 - DOI - PubMed
- Boulikas T. Putative nuclear-localization signals (NLS) in protein transcription factors. J Cell Biochem 1994; 55:32-58; http://dx.doi.org/10.1002/jcb.240550106 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources