Why infectious disease research needs community ecology - PubMed (original) (raw)
Review
Why infectious disease research needs community ecology
Pieter T J Johnson et al. Science. 2015.
Abstract
Infectious diseases often emerge from interactions among multiple species and across nested levels of biological organization. Threats as diverse as Ebola virus, human malaria, and bat white-nose syndrome illustrate the need for a mechanistic understanding of the ecological interactions underlying emerging infections. We describe how recent advances in community ecology can be adopted to address contemporary challenges in disease research. These analytical tools can identify the factors governing complex assemblages of multiple hosts, parasites, and vectors, and reveal how processes link across scales from individual hosts to regions. They can also determine the drivers of heterogeneities among individuals, species, and regions to aid targeting of control strategies. We provide examples where these principles have enhanced disease management and illustrate how they can be further extended.
Copyright © 2015, American Association for the Advancement of Science.
Figures
Fig. 1. The community ecology of infectious disease
(A to C) Co-infection by nematodes (A) increases host mortality due to bovine TB (B) among African buffalo (C) (63). (D to F) Tsimane villagers in Bolivia (D) reveal negative correlations between Giardia lamblia (E) and Ascaris lumbricoides (F), where deworming increased Giardia (99). (G and H) For tick-borne encephalitis (G), 93% of transmission events involve large-bodied, male yellow-necked mice (H), which constitute <20% of the population (53). (I and J) For humans, disproportionate contact among individuals (I) led to “superspreading events” for SARS (J) (50). (K to N) Among-species heterogeneities can alter community-wide transmission. Crayfish plague (K) introduced to Europe with highly susceptible red swamp crayfish (L) led to native crayfish declines; biodiversity losses tend to promote interactions between ticks and white-footed mice (M), which are highly competent hosts for Borrelia burgdorferi (N) and influence production of infected ticks that transmit Lyme borreliosis (65). [Image credits: [(A), (E), (I), (J)] CDC, (B) R. Grencis, (C) Y. Krishnappa, (D) A. Pisor, (F) F Dubs, (G) (100) (H) V. Dostál, (K) T. Vrålstad, (L) F Pupin, (M) J. Brunner, (N) NIH]
Fig. 2. Ecological hierarchies applied to host-parasite interactions and analogous processes in community ecology
The range of scales includes within-host (“parasite infracommunity,” often dominated by parasite-parasite and parasite-immune system interactions); between-host (“parasite component community,” population biology); among species (“parasite supracommunity,” community ecology); and across regions (macroecology and disease biogeography). The different colored squares represent different parasite species; the text at the right and left highlights the relevant processes from community ecology and disease ecology, respectively. The potential importance for interactions and feedback across these scales represents an essential research frontier in the field of disease community ecology.
Fig. 3. Parasite community assembly depends on a combination of ecological selection, ecological drift, and dispersal
(A) After input via dispersal (indicated as arrows from the parasite regional pool), parasite establishment depends on ecological selection: different species (mice versus prairie dogs) select for different parasites according to genetics, behavior, immune status, and other host properties (including vaccination status or drug presence). Dashed arrows indicate failed infection. Deterministic, within-host parasite interactions (indicated by + and − signs) are an additional niche-based influence on parasite communities; positive parasite interactions (facilitation) are indicated by solid arrows; negative interactions are indicated by dashed arrows. (B) Parasite community assembly is also influenced by ecological drift (stochasticity), particularly when colonizing populations are small or the outcome of parasite interactions depends on their order of arrival (“priority effects”). As a result, parasite communities can appear random with respect to host species or type, even if strongly affected by species interactions. (C) High rates of dispersal can swamp niche effects and overwhelm stochasticity, resulting in more similar parasite communities across hosts, regardless of host species. For simplicity, no feedback loops are shown from the individual hosts back to the parasite pool, although understanding such feedbacks represents an important research priority (Fig. 2).
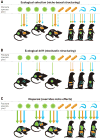
Fig. 4. How community ecology can inform infectious disease management
(A) Using community ecology–based management strategies for infectious disease. Levels of ecological organization are shown in the middle, and colored arrows indicate the ecological processes that connect these levels. Parasite dispersal connects scales going up through the hierarchy; parasite establishment connects scales moving down the hierarchy. Blue arrows indicate the relative importance of offensive strategies (preventing parasite dispersal) and defensive strategies (preventing parasite establishment), with darker shades reflecting greater importance. (B) Management strategies focused on reducing spillover from wildlife to humans (zoonosis) and from humans to wildlife (anthronosis or reverse zoonosis). Probability of spillover and subsequent spread of infection can be reduced through four major strategies: (i) Control may focus on reducing disease prevalence in reservoir hosts; for instance, vaccine baits have been successfully used to eliminate rabies from several European countries (80). (ii) Contact rates can be reduced between humans and wild animals (8); for example, limiting the proximity between humans and wildlife can reduce spillover of human illnesses such as measles, tuberculosis, and MRSA to wildlife. (iii) Zoonotic risk can be reduced by lowering the probability of infection when contact is unavoidable or unpredictable. For instance, some human dengue vaccine candidates provide cross-protection against sylvatic dengue viruses, which naturally circulate in nonhuman primates (85). (iv) When spillover does occur, regional control strategies—including isolation of infected populations, dispatching of medical personnel and aid, and enhanced border control—can be used to prevent disease transmission across borders.
Similar articles
- Towards an eco-phylogenetic framework for infectious disease ecology.
Fountain-Jones NM, Pearse WD, Escobar LE, Alba-Casals A, Carver S, Davies TJ, Kraberger S, Papeş M, Vandegrift K, Worsley-Tonks K, Craft ME. Fountain-Jones NM, et al. Biol Rev Camb Philos Soc. 2018 May;93(2):950-970. doi: 10.1111/brv.12380. Epub 2017 Nov 8. Biol Rev Camb Philos Soc. 2018. PMID: 29114986 - From within host dynamics to the epidemiology of infectious disease: Scientific overview and challenges.
Gutierrez JB, Galinski MR, Cantrell S, Voit EO. Gutierrez JB, et al. Math Biosci. 2015 Dec;270(Pt B):143-55. doi: 10.1016/j.mbs.2015.10.002. Epub 2015 Oct 16. Math Biosci. 2015. PMID: 26474512 Free PMC article. Review. - The Human Microbiota, Infectious Disease, and Global Health: Challenges and Opportunities.
Waldman AJ, Balskus EP. Waldman AJ, et al. ACS Infect Dis. 2018 Jan 12;4(1):14-26. doi: 10.1021/acsinfecdis.7b00232. Epub 2017 Dec 13. ACS Infect Dis. 2018. PMID: 29207239 - Diversity, decoys and the dilution effect: how ecological communities affect disease risk.
Johnson PT, Thieltges DW. Johnson PT, et al. J Exp Biol. 2010 Mar 15;213(6):961-70. doi: 10.1242/jeb.037721. J Exp Biol. 2010. PMID: 20190121 - Human drivers of ecological and evolutionary dynamics in emerging and disappearing infectious disease systems.
Rogalski MA, Gowler CD, Shaw CL, Hufbauer RA, Duffy MA. Rogalski MA, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Jan 19;372(1712):20160043. doi: 10.1098/rstb.2016.0043. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 27920388 Free PMC article. Review.
Cited by
- Host and parasite identity interact in scale-dependent fashion to determine parasite community structure.
Brian JI, Aldridge DC. Brian JI, et al. Oecologia. 2024 Jan;204(1):199-211. doi: 10.1007/s00442-023-05499-3. Epub 2024 Jan 11. Oecologia. 2024. PMID: 38206416 Free PMC article. - Effects of plant tissue permeability on invasion and population bottlenecks of a phytopathogen.
Jiang G, Zhang Y, Chen M, Ramoneda J, Han L, Shi Y, Peyraud R, Wang Y, Shi X, Chen X, Ding W, Jousset A, Hikichi Y, Ohnishi K, Zhao FJ, Xu Y, Shen Q, Dini-Andreote F, Zhang Y, Wei Z. Jiang G, et al. Nat Commun. 2024 Jan 2;15(1):62. doi: 10.1038/s41467-023-44234-7. Nat Commun. 2024. PMID: 38167266 Free PMC article. - Global Patterns of Zoonotic Disease in Mammals.
Han BA, Kramer AM, Drake JM. Han BA, et al. Trends Parasitol. 2016 Jul;32(7):565-577. doi: 10.1016/j.pt.2016.04.007. Epub 2016 Jun 14. Trends Parasitol. 2016. PMID: 27316904 Free PMC article. Review. - Bacterial biodiversity drives the evolution of CRISPR-based phage resistance.
Alseth EO, Pursey E, Luján AM, McLeod I, Rollie C, Westra ER. Alseth EO, et al. Nature. 2019 Oct;574(7779):549-552. doi: 10.1038/s41586-019-1662-9. Epub 2019 Oct 23. Nature. 2019. PMID: 31645729 Free PMC article. - High burdens of Ixodes scapularis larval ticks on white-tailed deer may limit Lyme disease risk in a low biodiversity setting.
Huang CI, Kay SC, Davis S, Tufts DM, Gaffett K, Tefft B, Diuk-Wasser MA. Huang CI, et al. Ticks Tick Borne Dis. 2019 Feb;10(2):258-268. doi: 10.1016/j.ttbdis.2018.10.013. Epub 2018 Nov 3. Ticks Tick Borne Dis. 2019. PMID: 30446377 Free PMC article.
References
- Sepúlveda J, Murray C. The state of global health in 2014. Science. 2014;345:1275–1278. - PubMed
- World Health Organization. The Top 10 Causes of Death. 2014 www.who.int/mediacentre/factsheets/fs310/en.
Publication types
MeSH terms
Grants and funding
- R01 GM109499/GM/NIGMS NIH HHS/United States
- R01 GM109501/GM/NIGMS NIH HHS/United States
- R01GM109499/GM/NIGMS NIH HHS/United States
- R01GM109501/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical