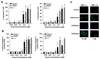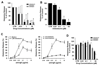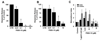Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth - PubMed (original) (raw)
doi: 10.1038/leu.2015.242. Epub 2015 Sep 9.
G E Winter 3, K Blatt 2, K L Bennett 3, G Stefanzl 2, U Rix 3 4, G Eisenwort 1, E Hadzijusufovic 1 2, M Gridling 3, C Dutreix 5, G Hoermann 6, J Schwaab 7, D Radia 8, J Roesel 5, P W Manley 5, A Reiter 7, G Superti-Furga 3, P Valent 1 2
Affiliations
- PMID: 26349526
- PMCID: PMC4896384
- DOI: 10.1038/leu.2015.242
Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth
B Peter et al. Leukemia. 2016 Feb.
Abstract
Proteomic-based drug testing is an emerging approach to establish the clinical value and anti-neoplastic potential of multikinase inhibitors. The multikinase inhibitor midostaurin (PKC412) is a promising new agent used to treat patients with advanced systemic mastocytosis (SM). We examined the target interaction profiles and the mast cell (MC)-targeting effects of two pharmacologically relevant midostaurin metabolites, CGP52421 and CGP62221. All three compounds, midostaurin and the two metabolites, suppressed IgE-dependent histamine secretion in basophils and MC with reasonable IC(50) values. Midostaurin and CGP62221 also produced growth inhibition and dephosphorylation of KIT in the MC leukemia cell line HMC-1.2, whereas the second metabolite, CGP52421, which accumulates in vivo, showed no substantial effects. Chemical proteomic profiling and drug competition experiments revealed that midostaurin interacts with KIT and several additional kinase targets. The key downstream regulator FES was recognized by midostaurin and CGP62221, but not by CGP52421 in MC lysates, whereas the IgE receptor downstream target SYK was recognized by both metabolites. Together, our data show that the clinically relevant midostaurin metabolite CGP52421 inhibits IgE-dependent histamine release, but is a weak inhibitor of MC proliferation, which may have clinical implications and may explain why mediator-related symptoms improve in SM patients even when disease progression occurs.
Conflict of interest statement
The other authors declare no other conflicts of interest in this study.
Figures
Figure 1. Effects of midostaurin, CGP52421 and CGP62221 on growth of neoplastic mast cells
A-C: HMC-1.1 cells (A, left panel) and HMC-1.2 cells (A, right panel) as well as primary bone marrow (BM) mononuclear cells obtained from 2 patients with indolent systemic mastocytosis (ISM; mast cell purity: 10% and 3%) (B) and 2 with aggressive SM (ASM; mast cell purity: 10% and 3%) (C); and peripheral blood MNC from one patient with mast cell leukemia (MCL; mast cell purity: 97%) (C), were incubated in control medium (CTR) or various concentrations of midostaurin, CGP52421 or CGP62221 as indicated at 37°C for 48 hours. After incubation, uptake of 3H-thymidine was measured. Results shown in “A” are expressed as percent of medium control (Co) and represent the mean±S.D. from 7 independent experiments. Results shown in “B” and “C” are expressed as percent of control and represent the mean±S.D. of triplicates.
Figure 2. Effects of midostaurin, CGP52421 and CGP62221 on viability of HMC-1 cells
A: HMC-1 cells were incubated in control medium (CTR) or in various concentrations of midostaurin, CGP52421 or CGP62221 at 37°C for 24 hours. Thereafter, the percentage of apoptotic cells was quantified by light microscopy. Results represent the mean±S.D. of 4 independent experiments. Asterisk: p<0.05. B: HMC-1 cells were incubated in control medium (CTR) or various concentrations of midostaurin or its metabolites at 37°C for 24 hours. Thereafter, cells were stained with an antibody against active caspase 3 and analyzed by flow cytometry. Results show the percentages of active caspase 3-positive cells and represent the mean±S.D. of 3 independent experiments. Asterisk: p<0.05. C: HMC-1 cells were incubated in control medium, midostaurin, CGP52421 or CGP62221 at 37°C for 24 hours. In HMC-1.2 cells (right panel), drugs were applied at 1 µM and in HMC-1.1 cells (left panel), drugs were applied at 0.5 µM. After incubation, cells were examined for viability and apoptosis by Tunel assay as described in the text.
Figure 3. Effects of midostaurin on histamine release in human basophils and MC
A: Primary blood basophils (healthy donors) were incubated in control medium (CTR) with or without midostaurin, CGP52421 or CGP62221 (0.01-10 µM) for 30 minutes. Thereafter, cells were exposed to histamine release buffer (HRB) with or without anti-IgE antibody E-124.2.8 (1 µg/ml) at 37°C for 30 minutes. After incubation, cells were centrifuged at 4°C, and cell-free supernatants and cell suspensions recovered and examined for histamine-content by RIA. Histamine release was calculated as percent of total histamine and is expressed as percent of control. Results represent the mean±S.D. of 5 independent experiments. Asterisk: p<0.05. B: Primary blood basophils (ISM=2, ASM=1) were incubated in control medium (CTR) with or without midostaurin (0.01-10 µM) for 30 minutes. Then, histamine release was measured as described above. Histamine release was calculated as percent of total histamine and is expressed as percent of control. Results represent the mean±S.D. of 3 independent experiments. Asterisk: p<0.05. C, left panel: Basophils from a patient with ASM (before therapy) were incubated in medium or medium containing 1 µM midostaurin at 37°C for 30 minutes. Thereafter, cells were incubated in HRB in the absence or presence of anti-IgE antibody E-124.2.8 (0.001-10 µg/ml) at 37°C for 30 minutes. After incubation, cells were centrifuged at 4°C, and cell-free supernatants and cell suspensions analyzed for histamine content. Histamine release is expressed as percent of total histamine; results represent the mean±S.D. of triplicates. C, right panel: Basophils obtained from the same patient with ASM before(?-?) and 8 days after (?-?) treatment with midostaurin (100 mg twice daily) were incubated in HRB in the absence or presence of anti-IgE antibody E-124.2.8 (0.001-10 µg/ml) for 30 minutes. Then, histamine release was measured as described above. Results represent the mean±S.D. of triplicates. D: Primary bone marrow MNC from a patient with ASM (purity of MC: 40%) were incubated in control medium (CTR) or medium containing midostaurin, CGP52421 or CGP62221 (0.01-10 µM) for 30 minutes. Thereafter, histamine release was measured. Histamine release was expressed as percent of control. Results represent the mean±S.D. of triplicates.
Figure 4. Midostaurin kinase target signature in HMC-1 cells and primary neoplastic mast cells (MC) as determined by chemical proteomic profiling (CPP)
Kinase target profiles obtained by CPP using c-midostaurin and lysates of HMC-1 cells (A), primary neoplastic cells (5% MC) of a patient with aggressive systemic mastocytosis (ASM) (B, left panel) and neoplastic MC (>95% purity) of a patient with MC leukemia (MCL) (B, right panel). HMC-1.1 cells, HMC-1.2 cells, and primary MC were processed and analyzed by CPP and liquid chromatography mass spectrometry (LCMS) as described in the text. The figure shows major kinase binders identified in form of a kinome-map adapted from Cell Signaling Technology (
). D: Drug affinity experiments were performed using HMC-1.2 cell lysates and various drugs applied to compete with c-midostaurin in its binding to FES. Expression of FES was determined by Western Blotting as described in the text.
Figure 5. Effects of SYK inhibition on histamine release on MC and CD63 and CD203c upregulation in basophils
A and B: Primary blood basophils (A) and cord blood derived MC (B) were incubated in control medium (CTR) with or without P505-15 (0.01-10 µM) at 37°C for 30 minutes. Thereafter, cells were exposed to HRB with or without anti-IgE antibody E-124.2.8 (1 µg/ml for basophils; 10 µg/ml for MC) at 37°C (30 minutes). After incubation, cells were centrifuged at 4°C, cell-free supernatants and cell suspensions recovered and examined for histamine content by RIA. Histamine release was calculated as percent of total histamine and is expressed as percent of control. Results represent the mean±S.D. of 4 (A) or 3 (B) independent experiments. Asterisk: p<0.05. C: Human basophils in whole blood were preincubated with medium (CTR) or medium containing P505-15 (0.01-10 µM) at 37°C for 30 minutes. Then cells were challenged with anti-IgE (1 µg/ml) at 37°C for 15 minutes. Thereafter, expression of CD63 and CD203c was analyzed by flow cytometry as described in the text. Upregulation of CD63 and CD203c is expressed as stimulation index (SI). Results represent the mean±S.D. of 3 independent experiments. Asterisk: p<0.05.
Similar articles
- Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells.
Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Krauth MT, et al. Clin Exp Allergy. 2009 Nov;39(11):1711-20. doi: 10.1111/j.1365-2222.2009.03353.x. Clin Exp Allergy. 2009. PMID: 19860818 - Midostaurin: a magic bullet that blocks mast cell expansion and activation.
Valent P, Akin C, Hartmann K, George TI, Sotlar K, Peter B, Gleixner KV, Blatt K, Sperr WR, Manley PW, Hermine O, Kluin-Nelemans HC, Arock M, Horny HP, Reiter A, Gotlib J. Valent P, et al. Ann Oncol. 2017 Oct 1;28(10):2367-2376. doi: 10.1093/annonc/mdx290. Ann Oncol. 2017. PMID: 28945834 Free PMC article. Review. - Drug-induced inhibition of phosphorylation of STAT5 overrides drug resistance in neoplastic mast cells.
Peter B, Bibi S, Eisenwort G, Wingelhofer B, Berger D, Stefanzl G, Blatt K, Herrmann H, Hadzijusufovic E, Hoermann G, Hoffmann T, Schwaab J, Jawhar M, Willmann M, Sperr WR, Zuber J, Sotlar K, Horny HP, Moriggl R, Reiter A, Arock M, Valent P. Peter B, et al. Leukemia. 2018 Apr;32(4):1016-1022. doi: 10.1038/leu.2017.338. Epub 2017 Nov 29. Leukemia. 2018. PMID: 29249817 Free PMC article. - Synergistic growth-inhibitory effects of ponatinib and midostaurin (PKC412) on neoplastic mast cells carrying KIT D816V.
Gleixner KV, Peter B, Blatt K, Suppan V, Reiter A, Radia D, Hadzijusufovic E, Valent P. Gleixner KV, et al. Haematologica. 2013 Sep;98(9):1450-7. doi: 10.3324/haematol.2012.079202. Epub 2013 Mar 28. Haematologica. 2013. PMID: 23539538 Free PMC article. - Midostaurin treatment in FLT3-mutated acute myeloid leukemia and systemic mastocytosis.
Kayser S, Levis MJ, Schlenk RF. Kayser S, et al. Expert Rev Clin Pharmacol. 2017 Nov;10(11):1177-1189. doi: 10.1080/17512433.2017.1387051. Epub 2017 Oct 10. Expert Rev Clin Pharmacol. 2017. PMID: 28960095 Review.
Cited by
- Mast Cell-Targeting Therapies in Mast Cell Activation Syndromes.
Sabato V, Beyens M, Toscano A, Van Gasse A, Ebo DG. Sabato V, et al. Curr Allergy Asthma Rep. 2024 Feb;24(2):63-71. doi: 10.1007/s11882-023-01123-9. Epub 2024 Jan 13. Curr Allergy Asthma Rep. 2024. PMID: 38217824 Review. - In silico repurposing of midostaurin as a therapeutic candidate for head and neck cancer via targeting SPARC/MMP9/CD44 Cancer-Associated Fibroblasts (CAFs) oncogenic signature.
Lin KC, Wu CC, Mokgautsi N, Lawal B, Wu CZ, Wu AT, Shen YK, Liu MC. Lin KC, et al. Am J Cancer Res. 2023 Mar 15;13(3):1004-1025. eCollection 2023. Am J Cancer Res. 2023. PMID: 37034220 Free PMC article. - Research Advances in Mast Cell Biology and Their Translation Into Novel Therapies for Anaphylaxis.
Dispenza MC, Metcalfe DD, Olivera A. Dispenza MC, et al. J Allergy Clin Immunol Pract. 2023 Jul;11(7):2032-2042. doi: 10.1016/j.jaip.2023.03.015. Epub 2023 Mar 21. J Allergy Clin Immunol Pract. 2023. PMID: 36958519 Free PMC article. Review. - Antineoplastic efficacy profiles of avapritinib and nintedanib in KIT D816V+ systemic mastocytosis: a preclinical study.
Degenfeld-Schonburg L, Gamperl S, Stefanzl G, Schruef AK, Sadovnik I, Bauer K, Smiljkovic D, Eisenwort G, Peter B, Greiner G, Hadzijusufovic E, Schwaab J, Sperr WR, Hoermann G, Kopanja S, Szépfalusi Z, Hoetzenecker K, Jaksch P, Reiter A, Arock M, Valent P. Degenfeld-Schonburg L, et al. Am J Cancer Res. 2023 Feb 15;13(2):355-378. eCollection 2023. Am J Cancer Res. 2023. PMID: 36895976 Free PMC article. - Human Lung Mast Cells: Therapeutic Implications in Asthma.
Poto R, Criscuolo G, Marone G, Brightling CE, Varricchi G. Poto R, et al. Int J Mol Sci. 2022 Nov 21;23(22):14466. doi: 10.3390/ijms232214466. Int J Mol Sci. 2022. PMID: 36430941 Free PMC article. Review.
References
- Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. - PubMed
- Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. - PubMed
- Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. - PubMed
- Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001;25:543–551. - PubMed
- Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F 4611/FWF_/Austrian Science Fund FWF/Austria
- F 4704/FWF_/Austrian Science Fund FWF/Austria
- F 4711/FWF_/Austrian Science Fund FWF/Austria
- P 21173/FWF_/Austrian Science Fund FWF/Austria
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous