Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress - PubMed (original) (raw)
Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress
Megumi Uetaki et al. Sci Rep. 2015.
Abstract
Intravenous administration of high-dose vitamin C has recently attracted attention as a cancer therapy. High-dose vitamin C induces pro-oxidant effects and selectively kills cancer cells. However, the anticancer mechanisms of vitamin C are not fully understood. Here, we analyzed metabolic changes induced by vitamin C in MCF7 human breast adenocarcinoma and HT29 human colon cancer cells using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS). The metabolomic profiles of both cell lines were dramatically altered after exposure to cytotoxic concentrations of vitamin C. Levels of upstream metabolites in the glycolysis pathway and tricarboxylic acid (TCA) cycle were increased in both cell lines following treatment with vitamin C, while adenosine triphosphate (ATP) levels and adenylate energy charges were decreased concentration-dependently. Treatment with N-acetyl cysteine (NAC) and reduced glutathione (GSH) significantly inhibited vitamin C-induced cytotoxicity in MCF7 cells. NAC also suppressed vitamin C-dependent metabolic changes, and NAD treatment prevented vitamin C-induced cell death. Collectively, our data suggests that vitamin C inhibited energy metabolism through NAD depletion, thereby inducing cancer cell death.
Figures
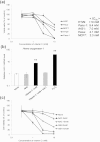
Figure 1. Effects of vitamin C-induced hydrogen peroxide (H2O2) on viability of cancer cells.
(a) Cancer cells were treated with vitamin C for 2 h, washed, and cultured for an additional 46 h in DMEM in the absence of vitamin C. Cell viability was determined using MTT assays. IC50 values indicate the concentration of vitamin C that inhibited survival by 50%, as determined by MTT assays. (b) Effects of vitamin C on HO-1 expression in MCF7 cells. Cells were treated with vitamin C (1 mM), NAC (10 mM), and H2O2 (1 mM) for 24 h. Expression levels of HO-1 mRNA were measured using qPCR. (c) Suppressive effects of antioxidants NAC and GSH on vitamin C-induced cytotoxicity in MCF7 cells. Cell viability was determined using MTT assays in MCF7 cells treated without or with vitamin C and antioxidants. Data are presented as means ± SDs from triplicate experiments, **P < 0.01.
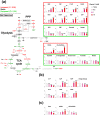
Figure 2. Vitamin C-induced metabolic alterations in MCF7 cells.
(a) Metabolic alterations in glycolysis and the TCA cycle induced by vitamin C. MCF7 cells were incubated in DMEM without or with vitamin C, and metabolites levels were measured using CE-TOSMS. Colors of metabolites on heatmap indicate significant differences (red, upregulated; green, downregulated). Bar graphs indicate fold changes relative to control sample (None). (b) Effects of vitamin C on levels of AMP, ADP, ATP, GMP, GDP, GTP, and adenylate energy charge. Bar graphs indicate fold changes relative to control sample (None). Adenylate energy charge calculation: (ATP + 0.5 × ADP)/(ATP + ADP + AMP). (c) Effects of vitamin C on levels of GSH and GSSG and GSH:GSSG ratio. Bar graphs show relative metabolite levels compared to control (None). Data are presented as means ± SD of triplicate experiments, *P < 0.05, **P < 0.01. ND, not detected.
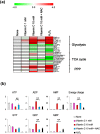
Figure 3. Effects of N-acetyl cysteine (NAC) on energy metabolism in MCF7 cells treated with vitamin C.
(a) Effects of NAC on metabolites of glycolysis, the TCA cycle, and the PPP in MCF7 cells stimulated with vitamin C. Heatmap depicts log2-transformed ratios of measured sample to control sample (None) concentrations. *P < 0.05, **P < 0.01 (comparing lanes 3 and 4). (b) Effects of NAC on levels of AMP, ADP, ATP, GMP, GDP, GTP, and adenylate energy charge. Bar graphs indicate fold changes relative to control sample (None). Adenylate energy charge calculation: (ATP + 0.5 × ADP)/(ATP + ADP + AMP). Data are presented as means ± SD of triplicate experiments. **P < 0.01.
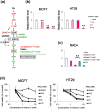
Figure 4. Nicotinamide adenine dinucleotide (NAD) depletion induced by vitamin C-induced H2O2 in MCF7 cells.
(a) Metabolite map of glycolysis. Colors of metabolites indicate significant differences (red, upregulated; green, downregulated). Conversion of GAP to 1,3- BPG mediated by GAPDH. (b) NAD levels were decreased by vitamin C in MCF7 (left) and HT29 cells (right) and were determined using CE-TOFMS. Bar graphs indicate fold changes relative to control sample (None). (c) Effects of NAC on levels of NAD in MCF7 cells. Bar graphs show metabolite levels relative to those of control (None). (d) Effects of NAD on viability of MCF7 (left) and HT29 (right) cells determined by MTT assay in both cell lines treated without or with vitamin C and NAD. Data are presented as means ± SD of triplicate experiments, **P < 0.01.
Similar articles
- Ascorbate kills breast cancer cells by rewiring metabolism via redox imbalance and energy crisis.
Ghanem A, Melzer AM, Zaal E, Neises L, Baltissen D, Matar O, Glennemeier-Marke H, Almouhanna F, Theobald J, Abu El Maaty MA, Berkers C, Wölfl S. Ghanem A, et al. Free Radic Biol Med. 2021 Feb 1;163:196-209. doi: 10.1016/j.freeradbiomed.2020.12.012. Epub 2020 Dec 23. Free Radic Biol Med. 2021. PMID: 33359260 - Vitamin B12b increases the cytotoxicity of short-time exposure to ascorbic acid, inducing oxidative burst and iron-dependent DNA damage.
Solovieva ME, Soloviev VV, Akatov VS. Solovieva ME, et al. Eur J Pharmacol. 2007 Jul 2;566(1-3):206-14. doi: 10.1016/j.ejphar.2007.03.035. Epub 2007 Mar 30. Eur J Pharmacol. 2007. PMID: 17475236 - Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry.
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Hirayama A, et al. Cancer Res. 2009 Jun 1;69(11):4918-25. doi: 10.1158/0008-5472.CAN-08-4806. Epub 2009 May 19. Cancer Res. 2009. PMID: 19458066 - Vitamin C as a Modulator of the Response to Cancer Therapy.
Blaszczak W, Barczak W, Masternak J, Kopczyński P, Zhitkovich A, Rubiś B. Blaszczak W, et al. Molecules. 2019 Jan 28;24(3):453. doi: 10.3390/molecules24030453. Molecules. 2019. PMID: 30695991 Free PMC article. Review. - Vitamin C in Cancer: A Metabolomics Perspective.
Park S, Ahn S, Shin Y, Yang Y, Yeom CH. Park S, et al. Front Physiol. 2018 Jun 19;9:762. doi: 10.3389/fphys.2018.00762. eCollection 2018. Front Physiol. 2018. PMID: 29971019 Free PMC article. Review.
Cited by
- Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications.
El Halabi I, Bejjany R, Nasr R, Mukherji D, Temraz S, Nassar FJ, El Darsa H, Shamseddine A. El Halabi I, et al. Int J Mol Sci. 2018 Sep 13;19(9):2752. doi: 10.3390/ijms19092752. Int J Mol Sci. 2018. PMID: 30217071 Free PMC article. Review. - The cytotoxicity effect of 7-hydroxy-3,4-dihydrocadalene from Heterotheca inuloides and semisynthetic cadalenes derivates towards breast cancer cells: involvement of oxidative stress-mediated apoptosis.
Mendoza-Fuentes A, González-Burgos E, Aparicio Trejo OE, Delgado-Lamas G, Rodríguez-Chávez JL, Pedraza-Chaverri J, Gómez-Serranillos MP, Araiza-Olivera D. Mendoza-Fuentes A, et al. PeerJ. 2023 Jun 20;11:e15586. doi: 10.7717/peerj.15586. eCollection 2023. PeerJ. 2023. PMID: 37361049 Free PMC article. - Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs).
De Francesco EM, Bonuccelli G, Maggiolini M, Sotgia F, Lisanti MP. De Francesco EM, et al. Oncotarget. 2017 Jun 9;8(40):67269-67286. doi: 10.18632/oncotarget.18428. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978032 Free PMC article. - Cocrystal Formation of Betulinic Acid and Ascorbic Acid: Synthesis, Physico-Chemical Assessment, Antioxidant, and Antiproliferative Activity.
Nicolov M, Ghiulai RM, Voicu M, Mioc M, Duse AO, Roman R, Ambrus R, Zupko I, Moaca EA, Coricovac DE, Farcas C, Racoviceanu RM, Danciu C, Dehelean CA, Soica C. Nicolov M, et al. Front Chem. 2019 Feb 21;7:92. doi: 10.3389/fchem.2019.00092. eCollection 2019. Front Chem. 2019. PMID: 30847340 Free PMC article. - TGF-β-dependent reprogramming of amino acid metabolism induces epithelial-mesenchymal transition in non-small cell lung cancers.
Nakasuka F, Tabata S, Sakamoto T, Hirayama A, Ebi H, Yamada T, Umetsu K, Ohishi M, Ueno A, Goto H, Sugimoto M, Nishioka Y, Yamada Y, Tomita M, Sasaki AT, Yano S, Soga T. Nakasuka F, et al. Commun Biol. 2021 Jun 24;4(1):782. doi: 10.1038/s42003-021-02323-7. Commun Biol. 2021. PMID: 34168290 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials