Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using LC/MS/MS - PubMed (original) (raw)
Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using LC/MS/MS
Qing Jiang et al. J Lipid Res. 2015 Nov.
Abstract
Tocopherols and tocotrienols are metabolized via hydroxylation and oxidation of their hydrophobic side chain to generate 13'-hydroxychromanols (13'-OHs) and various carboxychromanols, which can be further metabolized by conjugation including sulfation. Recent studies indicate that long-chain carboxychromanols, especially 13'-carboxychromanol (13'-COOH), appear to be more bioactive than tocopherols in anti-inflammatory and anticancer actions. To understand the potential contribution of metabolites to vitamin E-mediated effects, an accurate assay is needed to evaluate bioavailability of these metabolites. Here we describe an LC/MS/MS assay for quantifying vitamin E metabolites using negative polarity ESI. This assay includes a reliable sample extraction procedure with efficacy of ≥ 89% and interday/intraday variation of 3-11% for major metabolites. To ensure accurate quantification, short-chain, long-chain, and sulfated carboxychromanols are included as external/internal standards. Using this assay, we observed that sulfated carboxychromanols are the primary metabolites in the plasma of rodents fed with γ-tocopherol or δ-tocopherol. Although plasma levels of 13'-COOHs and 13'-OHs are low, high concentrations of these compounds are found in feces. Our study demonstrates an LC/MS/MS assay for quantitation of sulfated and unconjugated vitamin E metabolites, and this assay will be useful for evaluating the role of these metabolites in vivo.
Keywords: liquid chromatography tandem mass spectrometry; metabolism; sulfation; tocopherol; tocotrienol.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
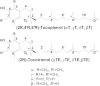
Fig. 1.
Structures of vitamin E forms.
Fig. 2.
Vitamin E (γT) metabolism and metabolites. Vitamin E forms are metabolized via ω-hydroxylation and oxidation to generate 13′-OH and 13′-COOH, which is subsequently metabolized via β-oxidation to form 11′-, 9′-, 7′-, 5′-, and 3′-COOH. Sulfation of intermediate carboxychromanols appears to take place in parallel with β-oxidation in response to supplementation of vitamin E forms.
Fig. 3.
LC/MS/MS fragmentation of vitamin E metabolites.
Similar articles
- Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats.
Jiang Q, Freiser H, Wood KV, Yin X. Jiang Q, et al. J Lipid Res. 2007 May;48(5):1221-30. doi: 10.1194/jlr.D700001-JLR200. Epub 2007 Feb 13. J Lipid Res. 2007. PMID: 17299205 Free PMC article. - Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism.
Parker RS, Sontag TJ, Swanson JE, McCormick CC. Parker RS, et al. Ann N Y Acad Sci. 2004 Dec;1031:13-21. doi: 10.1196/annals.1331.002. Ann N Y Acad Sci. 2004. PMID: 15753130 Review. - Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms.
Jiang Q. Jiang Q. IUBMB Life. 2019 Apr;71(4):495-506. doi: 10.1002/iub.1978. Epub 2018 Dec 11. IUBMB Life. 2019. PMID: 30548200 Review.
Cited by
- Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma.
Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, Yang C, Jenkins S, Wells H, Alexis N, Kesimer M, Bennett WD, Zhou H, Peden DB, Hernandez ML. Burbank AJ, et al. J Allergy Clin Immunol. 2018 Apr;141(4):1231-1238.e1. doi: 10.1016/j.jaci.2017.06.029. Epub 2017 Jul 20. J Allergy Clin Immunol. 2018. PMID: 28736267 Free PMC article. Clinical Trial. - Vitamin E metabolite 13'-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice.
Jang Y, Park NY, Rostgaard-Hansen AL, Huang J, Jiang Q. Jang Y, et al. Free Radic Biol Med. 2016 Jun;95:190-9. doi: 10.1016/j.freeradbiomed.2016.03.018. Epub 2016 Mar 23. Free Radic Biol Med. 2016. PMID: 27016075 Free PMC article. - High throughput profiling of tocochromanols in leaves and seeds of Arabidopsis and Maize.
Bao Y, Magallenes-Lundback M, Deason N, DellaPenna D. Bao Y, et al. Plant Methods. 2020 Sep 17;16:126. doi: 10.1186/s13007-020-00671-9. eCollection 2020. Plant Methods. 2020. PMID: 32968427 Free PMC article. - Simultaneous quantification of vitamin E and vitamin E metabolites in equine plasma and serum using LC-MS/MS.
Habib H, Finno CJ, Gennity I, Favro G, Hales E, Puschner B, Moeller BC. Habib H, et al. J Vet Diagn Invest. 2021 May;33(3):506-515. doi: 10.1177/10406387211005433. Epub 2021 Apr 13. J Vet Diagn Invest. 2021. PMID: 33847203 Free PMC article.
References
- Birringer M., Pfluger P., Kluth D., Landes N., Brigelius-Flohe R. 2002. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 132: 3113–3118. - PubMed
- Sontag T. J., Parker R. S. 2002. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277: 25290–25296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AT006882/AT/NCCIH NIH HHS/United States
- R01 ES023349/ES/NIEHS NIH HHS/United States
- P30CA023168/CA/NCI NIH HHS/United States
- UL1TR001108/TR/NCATS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- R01ES023349/ES/NIEHS NIH HHS/United States
- R01AT006882/AT/NCCIH NIH HHS/United States
- P30 CA023168/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical