Morphological divergence between three Arctic charr morphs - the significance of the deep-water environment - PubMed (original) (raw)
. 2015 Aug;5(15):3114-29.
doi: 10.1002/ece3.1573. Epub 2015 Jul 14.
Affiliations
- PMID: 26357540
- PMCID: PMC4559054
- DOI: 10.1002/ece3.1573
Morphological divergence between three Arctic charr morphs - the significance of the deep-water environment
Sigrid Skoglund et al. Ecol Evol. 2015 Aug.
Abstract
Morphological divergence was evident among three sympatric morphs of Arctic charr (Salvelinus alpinus (L.)) that are ecologically diverged along the shallow-, deep-water resource axis in a subarctic postglacial lake (Norway). The two deep-water (profundal) spawning morphs, a benthivore (PB-morph) and a piscivore (PP-morph), have evolved under identical abiotic conditions with constant low light and temperature levels in their deep-water habitat, and were morphologically most similar. However, they differed in important head traits (e.g., eye and mouth size) related to their different diet specializations. The small-sized PB-morph had a paedomorphic appearance with a blunt head shape, large eyes, and a deep body shape adapted to their profundal lifestyle feeding on submerged benthos from soft, deep-water sediments. The PP-morph had a robust head, large mouth with numerous teeth, and an elongated body shape strongly related to their piscivorous behavior. The littoral spawning omnivore morph (LO-morph) predominantly utilizes the shallow benthic-pelagic habitat and food resources. Compared to the deep-water morphs, the LO-morph had smaller head relative to body size. The LO-morph exhibited traits typical for both shallow-water benthic feeding (e.g., large body depths and small eyes) and planktivorous feeding in the pelagic habitat (e.g., streamlined body shape and small mouth). The development of morphological differences within the same deep-water habitat for the PB- and PP-morphs highlights the potential of biotic factors and ecological interactions to promote further divergence in the evolution of polymorphism in a tentative incipient speciation process. The diversity of deep-water charr in this study represents a novelty in the Arctic charr polymorphism as a truly deep-water piscivore morph has to our knowledge not been described elsewhere.
Keywords: Geometric morphometrics; incipient speciation; phenotypic diversity; profundal piscivore; resource polymorphism; salmonid.
Figures
Figure 1
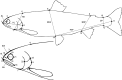
Color drawings showing the typical appearance of the three Arctic charr morphs from Skogsfjordvatnet: the littoral spawning omnivore morph (LO-morph), the profundal spawning piscivore morph (PP-morph), and the profundal spawning benthivore morph (PB-morph).
Figure 2
Landmark positions used in geometric morphometrics and measurements of linear morphological traits. Landmarks used for analyses of body shape: 1: Anterior point of the snout, 2: anterior extreme of bony orbit of the eye, 3: top of cranium at midpoint of eye, 4: top of cranium at posterior point of the bony opercle (5), 5: posterior point of the bony opercle, 6: dorsal insertion of pectoral fin, 7: anterioventral point of bony opercle, 8: anterior insertion of dorsal fin, 9: anterior base of adipose fin, 10: dorsal origin of caudal fin membrane, 11: posterior border of the hypural bones at the lateral midline, 12: ventral origin of caudal fin membrane, 13: anterior insertion of anal fin, and 14: anterior insertion of pelvic fin. Landmarks used for head shape: 1–7, and 15: Center of nostril, 16: top of cranium at midpoint of nostril (15), 17: anterior point of the upper jaw, 18: posterior point of the upper jaw, 19: ventral extreme of bony orbit of the eye, 20: dorsal extreme of bony orbit of the eye, 21: posterior extreme of bony orbit of the eye, 22: ventral point of intersection between the opercle and preopercle bones, 23: posterior point of intersection between the opercle and subopercle bones. Interlandmark distances used for linear morphological traits: caudal peduncle depth (CP): 10–12, body depth posterior (BSP): 9–13, body depth anterior (BDA): 8–14, postpelvic fin length (PPF): 11–14, head depth (HD): 4–7, head length (HL): 1–5, snout length (SL): 1–2, eye width (EW): 2–21, maxilla length (ML): 17–18.
Figure 3
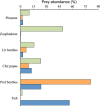
Diet (prey abundance, %) of the three Arctic charr morphs; the LO-morph (green bars), the PB-morph (orange bars), and the PP-morph (blue bars) from Skogsfjordvatn.
Figure 4
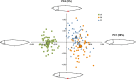
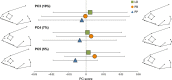
Principal component analysis of body shape (PC1 and PC4) in three morphs of Arctic charr. Mean values for each morph are illustrated by the larger symbols. Graphical illustrations show body shape at each extreme value on both axes (PC1: 0.05 and −0.05, PC4: 0.025 and −0.025). The red dot illustrates the position of the pelvic fin on extreme values of PC4.
Figure 5
Principal component analysis of head shape (PC1 and PC2) in three morphs of Arctic charr. Mean values for each morph are illustrated by the larger symbols. Graphical illustrations show head shape at each extreme value on both axes (PC1: 0.09 and −0.09, PC4: 0.07 and −0.07). The red line indicates the size of the upper maxilla on extreme values of PC2.
Figure 6
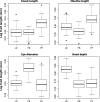
Box plots of size-corrected linear traits for the three Arctic charr morphs; the littoral spawning omnivore morph (LO-morph), the profundal spawning piscivore morph (PP-morph), and the profundal spawning benthivore morph (PB-morph).
Figure 7
Box plots illustrating differences between morphs (LO, PB, and PP) in four head traits accounting for differences in head size (length).
Figure 8
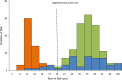
Length (in cm) distribution of the three different morphs (LO-morph in green, PB-morph in orange and PP-morph in blue) in Skogsfjordvatn with alignment size at 19.5 cm given.
Figure 9
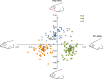
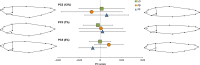
Mean and standard deviation for the three morphs of Arctic charr for PC2, 3, and 5 from a principal component analysis of body shape. Illustrations show the body shape associated with individual extreme values on PC2 (0.03 and −0.03), PC3 (0.03 and −0.03), and PC5 (0.025 and −0.025).
Figure 10
Mean and standard deviation for the three morphs of Arctic charr for PC3, PC4, and PC5 from a principal component analysis of head shape. Illustrations show the head shape associated with individual extreme values at 0.06 and −0.06 for all three PC axes.
Similar articles
- Allometric trajectories of body and head morphology in three sympatric Arctic charr (Salvelinus alpinus (L.)) morphs.
Simonsen MK, Siwertsson A, Adams CE, Amundsen PA, Præbel K, Knudsen R. Simonsen MK, et al. Ecol Evol. 2017 Aug 8;7(18):7277-7289. doi: 10.1002/ece3.3224. eCollection 2017 Sep. Ecol Evol. 2017. PMID: 28944016 Free PMC article. - The trade-off between fecundity and egg size in a polymorphic population of Arctic charr (Salvelinus alpinus (L.)) in Skogsfjordvatn, subarctic Norway.
Smalås A, Amundsen PA, Knudsen R. Smalås A, et al. Ecol Evol. 2017 Feb 26;7(7):2018-2024. doi: 10.1002/ece3.2669. eCollection 2017 Apr. Ecol Evol. 2017. PMID: 28405269 Free PMC article. - Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake.
Knudsen R, Klemetsen A, Amundsen PA, Hermansen B. Knudsen R, et al. Proc Biol Sci. 2006 Sep 22;273(1599):2291-8. doi: 10.1098/rspb.2006.3582. Proc Biol Sci. 2006. PMID: 16928630 Free PMC article. - Genetic Causes and Consequences of Sympatric Morph Divergence in Salmonidae: A Search for Mechanisms.
Salisbury SJ, Ruzzante DE. Salisbury SJ, et al. Annu Rev Anim Biosci. 2022 Feb 15;10:81-106. doi: 10.1146/annurev-animal-051021-080709. Epub 2021 Nov 10. Annu Rev Anim Biosci. 2022. PMID: 34758272 Review.
Cited by
- Parallelism in eco-morphology and gene expression despite variable evolutionary and genomic backgrounds in a Holarctic fish.
Jacobs A, Carruthers M, Yurchenko A, Gordeeva NV, Alekseyev SS, Hooker O, Leong JS, Minkley DR, Rondeau EB, Koop BF, Adams CE, Elmer KR. Jacobs A, et al. PLoS Genet. 2020 Apr 17;16(4):e1008658. doi: 10.1371/journal.pgen.1008658. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32302300 Free PMC article. - Phenotypic plasticity of a generalist fish species resident to lotic environments: Insights from the Great Lakes region.
Hetzel C, Forsythe P. Hetzel C, et al. Ecol Evol. 2023 Nov 20;13(11):e10715. doi: 10.1002/ece3.10715. eCollection 2023 Nov. Ecol Evol. 2023. PMID: 38020680 Free PMC article. - Variation in egg size and offspring phenotype among and within seven Arctic charr morphs.
Beck SV, Räsänen K, Kristjánsson BK, Skúlason S, Jónsson ZO, Tsinganis M, Leblanc CA. Beck SV, et al. Ecol Evol. 2022 Oct 18;12(10):e9427. doi: 10.1002/ece3.9427. eCollection 2022 Oct. Ecol Evol. 2022. PMID: 36267683 Free PMC article. - Ambient temperature as a factor contributing to the developmental divergence in sympatric salmonids.
Esin EV, Markevich GN, Melnik NO, Zlenko DV, Shkil FN. Esin EV, et al. PLoS One. 2021 Oct 15;16(10):e0258536. doi: 10.1371/journal.pone.0258536. eCollection 2021. PLoS One. 2021. PMID: 34653206 Free PMC article. - Allometric trajectories of body and head morphology in three sympatric Arctic charr (Salvelinus alpinus (L.)) morphs.
Simonsen MK, Siwertsson A, Adams CE, Amundsen PA, Præbel K, Knudsen R. Simonsen MK, et al. Ecol Evol. 2017 Aug 8;7(18):7277-7289. doi: 10.1002/ece3.3224. eCollection 2017 Sep. Ecol Evol. 2017. PMID: 28944016 Free PMC article.
References
- Adams CE, Fraser D, Huntingford FA, Greer R, Askew C. Walker F. Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. J. Fish Biol. 1998;52:1259–1271.
- Adams CE, Woltering C. Alexander G. Epigenetic regulation of trophic morphology through feeding behavior in Arctic charr, Salvelinus alpinus. Biol. J. Linn. Soc. 2003;78:43–49.
- Adams DC, Rohlf FJ. Slice DE. Geometric morphometrics: ten years of progress following the “Revolution”. Ital. J. Zool. 2004;71:5–16.
- Alekseyev SS. Pichugin MY. A new form of charr, Salvelinus alpinus (Salminidae) from Lake Davatchan in Transbaikalia and its morphological differences from sympatric forms. J. Ichthyol. 1998;38:328–337.
- Alekseyev SS, Samusenok V, Matveev A. Pichugin MY. Diversification, sympatric speciation, and trophic polymorphism of Arctic charr, Salvelinus alpinus complex, in Transbaikalia. Environ. Biol. Fishes. 2002;64:97–114.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous