Impact of an online writing aid tool for writing a randomized trial report: the COBWEB (Consort-based WEB tool) randomized controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
Impact of an online writing aid tool for writing a randomized trial report: the COBWEB (Consort-based WEB tool) randomized controlled trial
Caroline Barnes et al. BMC Med. 2015.
Abstract
Background: Incomplete reporting is a frequent waste in research. Our aim was to evaluate the impact of a writing aid tool (WAT) based on the CONSORT statement and its extension for non-pharmacologic treatments on the completeness of reporting of randomized controlled trials (RCTs).
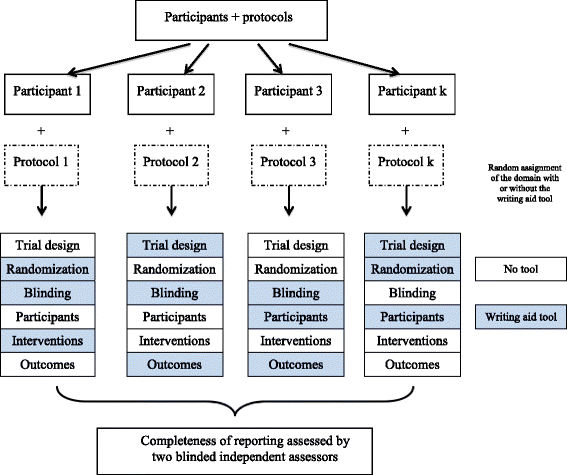
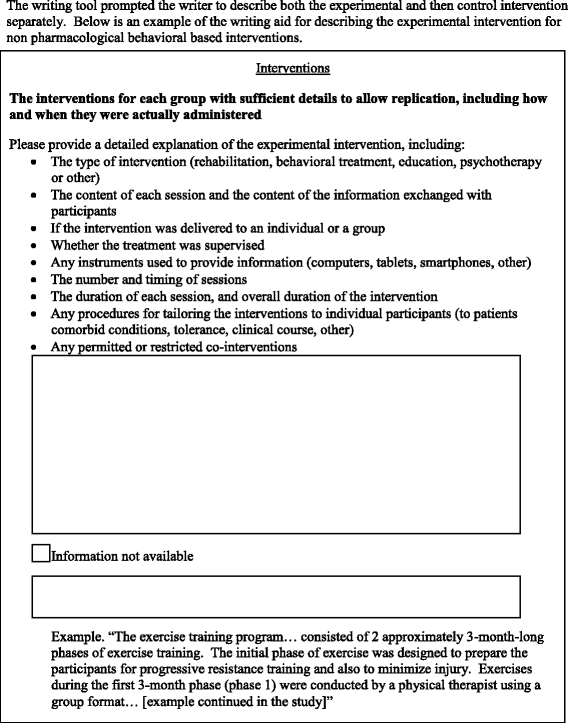
Methods: We performed a 'split-manuscript' RCT with blinded outcome assessment. Participants were masters and doctoral students in public health. They were asked to write, over a 4-hour period, the methods section of a manuscript based on a real RCT protocol, with a different protocol provided to each participant. Methods sections were divided into six different domains: 'trial design', 'randomization', 'blinding', 'participants', 'interventions', and 'outcomes'. Participants had to draft all six domains with access to the WAT for a random three of six domains. The random sequence was computer-generated and concealed. For each domain, the WAT comprised reminders of the corresponding CONSORT item(s), bullet points detailing all the key elements to be reported, and examples of good reporting. The control intervention consisted of no reminders. The primary outcome was the mean global score for completeness of reporting (scale 0-10) for all domains written with or without the WAT.
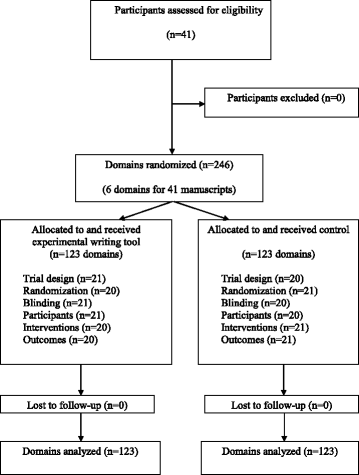
Results: Forty-one participants wrote 41 different manuscripts of RCT methods sections, corresponding to 246 domains (six for each of the 41 protocols). All domains were analyzed. For the primary outcome, the mean (SD) global score for completeness of reporting was higher with than without use of the WAT: 7.1 (1.2) versus 5.0 (1.6), with a mean (95 % CI) difference 2.1 (1.5-2.7; P <0.01). Completeness of reporting was significantly higher with the WAT for all domains except for blinding and outcomes.
Conclusion: Use of the WAT could improve the completeness of manuscripts reporting the results of RCTs.
Trial registration: Clinicaltrials.gov ( http://clinicaltrials.gov NCT02127567 , registration date first received April 29, 2014).
Figures
Fig. 1
Schema of the study design
Fig. 2
Example of the experimental writing tool
Fig. 3
Flow diagram of participants and domain randomization
Comment in
- A tool to make reporting checklists work.
Marušić A. Marušić A. BMC Med. 2015 Sep 28;13:243. doi: 10.1186/s12916-015-0476-3. BMC Med. 2015. PMID: 26412344 Free PMC article.
Similar articles
- There is no reliable evidence that providing authors with customized article templates including items from reporting guidelines improves completeness of reporting: the GoodReports randomized trial (GRReaT).
Struthers C, Harwood J, de Beyer JA, Logullo P, Collins GS. Struthers C, et al. BMC Med Res Methodol. 2025 Mar 14;25(1):71. doi: 10.1186/s12874-025-02518-0. BMC Med Res Methodol. 2025. PMID: 40087548 Free PMC article. Clinical Trial. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - A tool to make reporting checklists work.
Marušić A. Marušić A. BMC Med. 2015 Sep 28;13:243. doi: 10.1186/s12916-015-0476-3. BMC Med. 2015. PMID: 26412344 Free PMC article. - Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials.
Speich B, Mann E, Schönenberger CM, Mellor K, Griessbach AN, Dhiman P, Gandhi P, Lohner S, Agarwal A, Odutayo A, Puebla I, Clark A, Chan AW, Schlussel MM, Ravaud P, Moher D, Briel M, Boutron I, Schroter S, Hopewell S. Speich B, et al. JAMA Netw Open. 2023 Jun 1;6(6):e2317651. doi: 10.1001/jamanetworkopen.2023.17651. JAMA Netw Open. 2023. PMID: 37294569 Free PMC article. - The REFLECT statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration.
Sargeant JM, O'Connor AM, Gardner IA, Dickson JS, Torrence ME, Dohoo IR, Lefebvre SL, Morley PS, Ramirez A, Snedeker K. Sargeant JM, et al. J Food Prot. 2010 Mar;73(3):579-603. doi: 10.4315/0362-028x-73.3.579. J Food Prot. 2010. PMID: 20202349
Cited by
- Completeness of reporting in abstracts of randomized controlled trials in subscription and open access journals: cross-sectional study.
Jerčić Martinić-Cezar I, Marušić A. Jerčić Martinić-Cezar I, et al. Trials. 2019 Dec 2;20(1):669. doi: 10.1186/s13063-019-3781-x. Trials. 2019. PMID: 31791393 Free PMC article. - Reporting and transparent research practices in sports medicine and orthopaedic clinical trials: a meta-research study.
Schulz R, Langen G, Prill R, Cassel M, Weissgerber TL. Schulz R, et al. BMJ Open. 2022 Aug 8;12(8):e059347. doi: 10.1136/bmjopen-2021-059347. BMJ Open. 2022. PMID: 35940834 Free PMC article. - There is no reliable evidence that providing authors with customized article templates including items from reporting guidelines improves completeness of reporting: the GoodReports randomized trial (GRReaT).
Struthers C, Harwood J, de Beyer JA, Logullo P, Collins GS. Struthers C, et al. BMC Med Res Methodol. 2025 Mar 14;25(1):71. doi: 10.1186/s12874-025-02518-0. BMC Med Res Methodol. 2025. PMID: 40087548 Free PMC article. Clinical Trial. - Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, Catalá-López F, Li L, Reid EK, Sarkis-Onofre R, Moher D. Page MJ, et al. PLoS Med. 2016 May 24;13(5):e1002028. doi: 10.1371/journal.pmed.1002028. eCollection 2016 May. PLoS Med. 2016. PMID: 27218655 Free PMC article. - Effect of an editorial intervention to improve the completeness of reporting of randomised trials: a randomised controlled trial.
Blanco D, Schroter S, Aldcroft A, Moher D, Boutron I, Kirkham JJ, Cobo E. Blanco D, et al. BMJ Open. 2020 May 18;10(5):e036799. doi: 10.1136/bmjopen-2020-036799. BMJ Open. 2020. PMID: 32430454 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous