Genome Engineering Using Adeno-associated Virus: Basic and Clinical Research Applications - PubMed (original) (raw)
Review
Genome Engineering Using Adeno-associated Virus: Basic and Clinical Research Applications
Thomas Gaj et al. Mol Ther. 2016 Mar.
Abstract
In addition to their broad potential for therapeutic gene delivery, adeno-associated virus (AAV) vectors possess the innate ability to stimulate homologous recombination in mammalian cells at high efficiencies. This process--referred to as AAV-mediated gene targeting--has enabled the introduction of a diverse array of genomic modifications both in vitro and in vivo. With the recent emergence of targeted nucleases, AAV-mediated genome engineering is poised for clinical translation. Here, we review key properties of AAV vectors that underscore its unique utility in genome editing. We highlight the broad range of genome engineering applications facilitated by this technology and discuss the strong potential for unifying AAV with targeted nucleases for next-generation gene therapy.
Figures
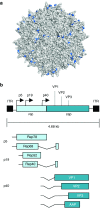
Figure 1
Adeno-associated virus (AAV) structure and genome organization. (a) Surface representation of the AAV2 capsid structure. The residues important for heparin binding, Arg 484, Arg 487, Lys 532, Arg 585, and Arg 588, are colored blue (PDB ID: 1LP3). (b) Structure of the wild-type AAV genome. Rep78 and Rep68 are expressed from the p5 promoter, and Rep52 and Rep40 are expressed from the p19 promoter. VP1, 2, 3, and the assembly-activating protein (AAP) are translated from the p40 transcript encoded by the cap gene. Solid black boxes indicate the inverted terminal repeats (ITRs).
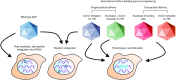
Figure 2
AAV integration into the human genome. Wild-type AAV vectors encoding the rep gene can facilitate AAV integration into a region of human chromosome 19 termed AAVS1, denoted by a blue circle. Wild-type and recombinant AAV vectors can also integrate into random chromosomal sites via nonhomologous end joining (denoted by blue circles). When the AAV vector genome is modified to contain a genomic sequence homologous to a specific chromosomal site, homologous recombination (HR) between the AAV vector and target site can occur (denoted by blue DNA fragment). Co-delivering a targeted nuclease within the same or a separate AAV particle can further enhance the frequency of HR.
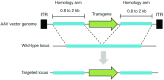
Figure 3
Overview of AAV-mediated gene targeting. AAV vectors containing DNA sequences homologous to a specific chromosomal site can be recombined with the matching genomic locus. By modifying the DNA sequence between the homology arms, targeted modifications (i.e., transgenes, single-base substitutions) can be introduced into the host genome. Dashes denote homologous regions of DNA. Black boxes indicate inverted terminal repeats (ITRs).
Similar articles
- Basic and Clinical Application of Adeno-Associated Virus-Mediated Genome Editing.
He X, Xie H, Liu X, Gu F. He X, et al. Hum Gene Ther. 2019 Jun;30(6):673-681. doi: 10.1089/hum.2018.190. Epub 2019 Feb 28. Hum Gene Ther. 2019. PMID: 30588843 Review. - Genome engineering using Adeno-Associated Virus (AAV).
Howes R, Schofield C. Howes R, et al. Methods Mol Biol. 2015;1239:75-103. doi: 10.1007/978-1-4939-1862-1_5. Methods Mol Biol. 2015. PMID: 25408402 - A multifunctional AAV-CRISPR-Cas9 and its host response.
Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, Zhu K, Wagers AJ, Church GM. Chew WL, et al. Nat Methods. 2016 Oct;13(10):868-74. doi: 10.1038/nmeth.3993. Epub 2016 Sep 5. Nat Methods. 2016. PMID: 27595405 Free PMC article. - Adeno-Associated Virus-Mediated Delivery of CRISPR-Cas Systems for Genome Engineering in Mammalian Cells.
Gaj T, Schaffer DV. Gaj T, et al. Cold Spring Harb Protoc. 2016 Nov 1;2016(11):10.1101/pdb.prot086868. doi: 10.1101/pdb.prot086868. Cold Spring Harb Protoc. 2016. PMID: 27803249 Free PMC article. - AAV Vectorization of DSB-mediated Gene Editing Technologies.
Moser RJ, Hirsch ML. Moser RJ, et al. Curr Gene Ther. 2016;16(3):207-19. doi: 10.2174/1566523216666160602213738. Curr Gene Ther. 2016. PMID: 27280971 Review.
Cited by
- Gene therapies that restore dystrophin expression for the treatment of Duchenne muscular dystrophy.
Robinson-Hamm JN, Gersbach CA. Robinson-Hamm JN, et al. Hum Genet. 2016 Sep;135(9):1029-40. doi: 10.1007/s00439-016-1725-z. Epub 2016 Aug 20. Hum Genet. 2016. PMID: 27542949 Free PMC article. Review. - Deep Parallel Characterization of AAV Tropism and AAV-Mediated Transcriptional Changes via Single-Cell RNA Sequencing.
Brown D, Altermatt M, Dobreva T, Chen S, Wang A, Thomson M, Gradinaru V. Brown D, et al. Front Immunol. 2021 Oct 21;12:730825. doi: 10.3389/fimmu.2021.730825. eCollection 2021. Front Immunol. 2021. PMID: 34759919 Free PMC article. - Concise Review: The Potential Use of Intestinal Stem Cells to Treat Patients with Intestinal Failure.
Hong SN, Dunn JC, Stelzner M, Martín MG. Hong SN, et al. Stem Cells Transl Med. 2017 Feb;6(2):666-676. doi: 10.5966/sctm.2016-0153. Epub 2016 Sep 16. Stem Cells Transl Med. 2017. PMID: 28191783 Free PMC article. Review. - CRISPR-Cas9-Mediated Genome Editing Increases Lifespan and Improves Motor Deficits in a Huntington's Disease Mouse Model.
Ekman FK, Ojala DS, Adil MM, Lopez PA, Schaffer DV, Gaj T. Ekman FK, et al. Mol Ther Nucleic Acids. 2019 Sep 6;17:829-839. doi: 10.1016/j.omtn.2019.07.009. Epub 2019 Jul 26. Mol Ther Nucleic Acids. 2019. PMID: 31465962 Free PMC article. - Recombinant AAV Integration Is Not Associated With Hepatic Genotoxicity in Nonhuman Primates and Patients.
Gil-Farina I, Fronza R, Kaeppel C, Lopez-Franco E, Ferreira V, D'Avola D, Benito A, Prieto J, Petry H, Gonzalez-Aseguinolaza G, Schmidt M. Gil-Farina I, et al. Mol Ther. 2016 Jun;24(6):1100-1105. doi: 10.1038/mt.2016.52. Epub 2016 Mar 7. Mol Ther. 2016. PMID: 26948440 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM098218/GM/NIGMS NIH HHS/United States
- R01EY022975/EY/NEI NIH HHS/United States
- F32 GM113446/GM/NIGMS NIH HHS/United States
- R01 EY022975/EY/NEI NIH HHS/United States
- T32 GM008155/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources