Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs - PubMed (original) (raw)
Review
Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs
Melissa Gabbs et al. Adv Nutr. 2015.
Abstract
Oxylipins formed from polyunsaturated fatty acids (PUFAs) are the main mediators of PUFA effects in the body. They are formed via cyclooxygenase, lipoxygenase, and cytochrome P450 pathways, resulting in the formation of prostaglandins, thromboxanes, mono-, di-, and tri-hydroxy fatty acids (FAs), epoxy FAs, lipoxins, eoxins, hepoxilins, resolvins, protectins (also called neuroprotectins in the brain), and maresins. In addition to the well-known eicosanoids derived from arachidonic acid, recent developments in lipidomic methodologies have raised awareness of and interest in the large number of oxylipins formed from other PUFAs, including those from the essential FAs and the longer-chain n-3 (ω-3) PUFAs. Oxylipins have essential roles in normal physiology and function, but can also have detrimental effects. Compared with the oxylipins derived from n-3 PUFAs, oxylipins from n-6 PUFAs generally have greater activity and more inflammatory, vasoconstrictory, and proliferative effects, although there are notable exceptions. Because PUFA composition does not necessarily reflect oxylipin composition, comprehensive analysis of the oxylipin profile is necessary to understand the overall physiologic effects of PUFAs mediated through their oxylipins. These analyses should include oxylipins derived from linoleic and α-linolenic acids, because these largely unexplored bioactive oxylipins constitute more than one-half of oxylipins present in tissues. Because collated information on oxylipins formed from different PUFAs is currently unavailable, this review provides a detailed compilation of the main oxylipins formed from PUFAs and describes their functions. Much remains to be elucidated in this emerging field, including the discovery of more oxylipins, and the understanding of the differing biological potencies, kinetics, and isomer-specific activities of these novel PUFA metabolites.
Keywords: cyclooxygenase; cytochrome P450; eicosanoid; lipid mediators; lipidomics; lipooxygenase; omega-3; omega-6; oxylipin; polyunsaturated fatty acid.
© 2015 American Society for Nutrition.
Conflict of interest statement
Author disclosures: M Gabbs, S Leng, JG Devassy, M Monirujjaman, and HM Aukema, no conflicts of interest.
Figures
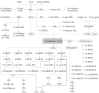
FIGURE 1
Arachidonic acid–derived oxylipins. There is also evidence for thromboxane synthase–independent production of HHTrE (416). 11-HETE and 15-HETE are also produced via the COX pathway (24, 25). ASA, acetylsalicylic acid; COX, cyclooxygenase; CYP, cytochrome P450; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpETrE, epoxy-eicosatrienoic acid; Ex, eoxin; HETE, hydroxy-eicosatetraenoic acid; HHTrE, hydroxy-heptadecatrienoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; Hx, hepoxilin; LOX, lipoxygenase; Lt, Leukotriene; Lx, lipoxin; oxo-ETE, oxo-eicosatetraenoic acid; PGEM, prostaglandin E metabolite; Trx, trioxilin; Tx, thromboxane.
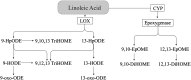
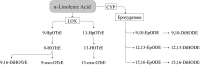
FIGURE 2
Linoleic acid–derived oxylipins. 9-HODE and 13-HODE are also produced via the COX pathway (27, 61). COX, cyclooxygenase; CYP, cytochrome P450; DiHOME, dihydroxy-octadecenoic acid; EpOME, epoxy-octadecenoic acid; HODE, hydroxy-octadecadienoic acid; HpODE, hydroperoxy-octadecadienoic acid; LOX, lipoxygenase; oxo-ODE, oxo-octadecadienoic acid; TriHOME, trihydroxy-octadecenoic acid.
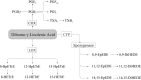
FIGURE 3
Dihomo-γ-linolenic acid–derived oxylipins. COX, cyclooxygenase; CYP, cytochrome P450; DiHEDE, dihydroxy-eicosadienoic acid; EpEDE, epoxy-eicosadienoic acid; HETrE, hydroxy-eicosatrienoic acid; HpETrE, hydroperoxy-eicosatrienoic acid; LOX, lipoxygenase; Tx, thromboxane.
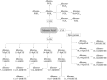
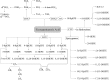
FIGURE 4
Adrenic acid–derived oxylipins. Dihomo-7,14-DiHETE and dihomo-7,17-DiHETE also can be formed from dihomo-7-HETE (76). COX, cyclooxygenase; CYP, cytochrome P450; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpETrE, epoxy-eicosatrienoic acid; HETE, hydroxy-eicosatetraenoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; LOX, lipoxygenase; Tx, thromboxane.
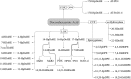
FIGURE 5
α-Linolenic acid–derived oxylipins. CYP, cytochrome P450; DiHODE, dihydroxy-octadecadienoic acid; DiHOTrE, dihydroxy-octadecatrienoic acid; EpODE, epoxy-octadecadienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HpOTrE, hydroperoxy-octadecatrienoic acid; LOX, lipoxygenase; oxo-OTrE, oxo-octadecatrienoic acid.
FIGURE 6
EPA-derived oxylipins. ASA, acetylsalicylic acid; COX, cyclooxygenase; CYP, cytochrome P450; DiHEPE, dihydroxy-eicosapentaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; EpETE, epoxy-eicosatetraenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HpEPE, hydroperoxy-eicosapentaenoic acid; LOX, lipoxygenase; Lt, Leukotriene; Lx, lipoxin; oxo-EPE, oxo-eicosapentaenoic acid; Rv, resolvin; Tx, thromboxane.
FIGURE 7
DHA-derived oxylipins. 13-HDoHE also is produced via the COX pathway (26). 14,21-DiHDoHE also may be formed from 21-HDoHE (313, 314). ASA, acetylsalicylic acid; AT, aspirin-triggered; COX, cyclooxygenase; CYP, cytochrome P450; DiHDoHE, dihydroxy-docosahexaenoic acid; DiHDPE, dihydroxy-docosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HpDoHE, hydroperoxy-docosahexaenoic acid; LOX, lipoxygenase; MaR, maresin; oxo-DoHE, oxo-docosahexaenoic acid; PD, protectin; Rv, resolvin.
Similar articles
- Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions.
Dyall SC, Balas L, Bazan NG, Brenna JT, Chiang N, da Costa Souza F, Dalli J, Durand T, Galano JM, Lein PJ, Serhan CN, Taha AY. Dyall SC, et al. Prog Lipid Res. 2022 Apr;86:101165. doi: 10.1016/j.plipres.2022.101165. Epub 2022 May 1. Prog Lipid Res. 2022. PMID: 35508275 Free PMC article. Review. - Synthesis and function of fatty acids and oxylipins, with a focus on Caenorhabditis elegans.
Mokoena NZ, Sebolai OM, Albertyn J, Pohl CH. Mokoena NZ, et al. Prostaglandins Other Lipid Mediat. 2020 Jun;148:106426. doi: 10.1016/j.prostaglandins.2020.106426. Epub 2020 Feb 4. Prostaglandins Other Lipid Mediat. 2020. PMID: 32032704 Review. - Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain.
Rey C, Delpech JC, Madore C, Nadjar A, Greenhalgh AD, Amadieu C, Aubert A, Pallet V, Vaysse C, Layé S, Joffre C. Rey C, et al. Brain Behav Immun. 2019 Feb;76:17-27. doi: 10.1016/j.bbi.2018.07.025. Epub 2018 Aug 4. Brain Behav Immun. 2019. PMID: 30086401 - The Brain Oxylipin Profile Is Resistant to Modulation by Dietary n-6 and n-3 Polyunsaturated Fatty Acids in Male and Female Rats.
Ferdouse A, Leng S, Winter T, Aukema HM. Ferdouse A, et al. Lipids. 2019 Jan;54(1):67-80. doi: 10.1002/lipd.12122. Epub 2019 Jan 29. Lipids. 2019. PMID: 30697757 - Oxidation of polyunsaturated fatty acids to produce lipid mediators.
Christie WW, Harwood JL. Christie WW, et al. Essays Biochem. 2020 Sep 23;64(3):401-421. doi: 10.1042/EBC20190082. Essays Biochem. 2020. PMID: 32618335 Free PMC article. Review.
Cited by
- Efficacy of Flaxseed Compared to ACE Inhibition in Treating Anthracycline- and Trastuzumab-Induced Cardiotoxicity.
Telles-Langdon SM, Arya V, Haasbeek PR, Cheung DYC, Eekhoudt CR, Mackic L, Bryson AN, Varghese SS, Austria JA, Thliveris JA, Aukema HM, Ravandi A, Singal PK, Jassal DS. Telles-Langdon SM, et al. CJC Open. 2024 Mar 25;6(7):925-937. doi: 10.1016/j.cjco.2024.03.009. eCollection 2024 Jul. CJC Open. 2024. PMID: 39026621 Free PMC article. - Oxylipins Associated with D3-Creatine Muscle Mass/Weight and Physical Performance among Community-Dwelling Older Men.
Marron MM, Orwoll ES, Cawthon PM, Lane NE, Newman AB, Cauley JA; Osteoporotic Fractures in Men (MrOS) Study Research Group. Marron MM, et al. Int J Mol Sci. 2022 Oct 25;23(21):12857. doi: 10.3390/ijms232112857. Int J Mol Sci. 2022. PMID: 36361650 Free PMC article. - Plasma Levels of Omega-3 and Omega-6 Derived Oxylipins Are Associated with Fecal Microbiota Composition in Young Adults.
Xu H, Jurado-Fasoli L, Ortiz-Alvarez L, Osuna-Prieto FJ, Kohler I, Di X, Vilchez-Vargas R, Link A, Plaza-Díaz J, Gil A, Rensen PCN, Ruiz JR, Martinez-Tellez B. Xu H, et al. Nutrients. 2022 Nov 24;14(23):4991. doi: 10.3390/nu14234991. Nutrients. 2022. PMID: 36501021 Free PMC article. - The Impact of Kidney Transplantation on the Serum Fatty Acid Profile in Patients with End-Stage Kidney Disease.
Śledziński M, Hliwa A, Gołębiewska J, Mika A. Śledziński M, et al. Nutrients. 2022 Feb 12;14(4):772. doi: 10.3390/nu14040772. Nutrients. 2022. PMID: 35215422 Free PMC article. - Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota.
Arreaza-Gil V, Ávila-Román J, Escobar-Martínez I, Muguerza B, Suárez M, Arola-Arnal A, Torres-Fuentes C. Arreaza-Gil V, et al. Nutrients. 2023 Jan 30;15(3):707. doi: 10.3390/nu15030707. Nutrients. 2023. PMID: 36771413 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous