Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients - PubMed (original) (raw)
Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients
Hagai Yanai et al. Aging (Albany NY). 2015 Sep.
Erratum in
- Aging (Albany NY). 2015 Nov;7(11):1022. Zeische, Rolf [corrected to Ziesche, Rolf]
Abstract
Idiopathic pulmonary fibrosis (IPF) is an age-related fatal disease with unknown etiology and no effective treatment. In this study, we show that primary cultures of fibroblasts derived from lung biopsies of IPF patients exhibited (i) accelerated replicative cellular senescence (CS); (ii) high resistance to oxidative-stress-induced cytotoxicity or CS; (iii) a CS-like morphology (even at the proliferative phase); and (iv) rapid accumulation of senescent cells expressing the myofibroblast marker α-SMA. Our findings suggest that CS could serve as a bridge connecting lung aging and its quite frequent outcome -- pulmonary fibrosis, and be an important player in the disease progression. Consequently, targeting senescent cells offers the potential of being a promising therapeutic approach.
Keywords: aging; cellular senescence; fibroblasts; idiopathic pulmonary fibrosis; myofibroblasts.
Conflict of interest statement
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
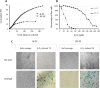
Figure 1. Growth curves and response to oxidative stress of IPF-derived and normal human pulmonary fibroblasts
(A) Lung fibroblasts derived from IPF patients (IPF-PF) and healthy subjects (N-PF) during routine culture. PD stands for Population Doublings. Note that IPF-derived fibroblasts cease to proliferate after passage 16, whereas the N-PF ones are still in the logarithmic phase of cell growth. The difference between N-PF and IPF-PF growth curves is highly significant (Mann–Whitney _U_-test; p < 0.001). (B) Lung fibroblasts derived from IPF patients (IPF-PF) and healthy (N-PF) were treated with indicated doses of H2O2 for two hours, and tested for viability by Neutral Red assay (LD50 was 28.7 μM and 136 μM for N-PF and IPF-PF, respectively; p < E-06). Results represent 3 independent experiments. (C) SA-β-gal staining of normal (left panel) and IPF-derived fibroblasts (right panel) treated with H2O2 (the doses that caused 20% cytotoxicity were used).
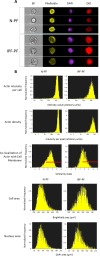
Figure 2. Morphology analysis of lung fibroblasts by ImageStreamX
BF – bright field. Phalloidin, DAPI and DiD refer to staining of actin, nucleus and cell membrane, respectively. (A) Selected representative images. (B) Distribution of cell populations according to indicated criterion. The meaning of each variable is explained next to the images. Red lines indicate the cells in which actin is co-localized with cell membrane (score > 1.5). Note: IPF-PF vs. N-PF represent a more heterogeneous (more variable) cell population as evident by CV values (Table 1).
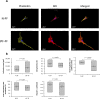
Figure 3. Morphology analysis of lung fibroblasts by Z-stack confocal microscopy
(A) Representative image of lung fibroblasts derived from IPF patients (IPF-PF) and healthy (N-PF). Background subtracted. Phalloidin stains for actin; DiD for membrane and all pictures show DAPI staining of the nucleus (B) Quantification of cell morphology using the Imaris software (see methods) of confocal z-stack images taken for fibroblasts of early passage (passage 7). Whiskers indicate standard error [; bars indicate range (min/max); middle line indicates mean.
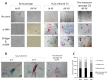
Figure 4. Immunostaining for SA-β-gal and α-SMA in primary cultures of pulmonary fibroblasts
(A) early passages (upper left panels) and at cellular senescence (upper right panels). SA-β-gal – green staining; α-SMA – red staining. (B) Representative co-stained cells. (C) Distribution of cells expressing α-SMA, SA-β-gal, or both in senescent primary cultures of normal pulmonary (N-PF), IPF-derived fibroblasts (IPF-PF) and normal dermal Fibroblasts (HDF). The difference between the two pulmonary cell types was insignificant (p > 0.05).
Similar articles
- Regulation of Cellular Senescence Is Independent from Profibrotic Fibroblast-Deposited ECM.
Blokland KEC, Habibie H, Borghuis T, Teitsma GJ, Schuliga M, Melgert BN, Knight DA, Brandsma CA, Pouwels SD, Burgess JK. Blokland KEC, et al. Cells. 2021 Jun 29;10(7):1628. doi: 10.3390/cells10071628. Cells. 2021. PMID: 34209854 Free PMC article. - Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes.
Kanaji N, Basma H, Nelson A, Farid M, Sato T, Nakanishi M, Wang X, Michalski J, Li Y, Gunji Y, Feghali-Bostwick C, Liu X, Rennard SI. Kanaji N, et al. Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L364-73. doi: 10.1152/ajplung.00041.2014. Epub 2014 Jul 11. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25015975 - Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo.
Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr, Hogaboam CM. Hohmann MS, et al. Am J Respir Cell Mol Biol. 2019 Jan;60(1):28-40. doi: 10.1165/rcmb.2017-0289OC. Am J Respir Cell Mol Biol. 2019. PMID: 30109946 Free PMC article. - Cell senescence and fibrotic lung diseases.
Liu RM, Liu G. Liu RM, et al. Exp Gerontol. 2020 Apr;132:110836. doi: 10.1016/j.exger.2020.110836. Epub 2020 Jan 17. Exp Gerontol. 2020. PMID: 31958492 Free PMC article. Review. - Senotherapeutics: Targeting senescence in idiopathic pulmonary fibrosis.
Merkt W, Bueno M, Mora AL, Lagares D. Merkt W, et al. Semin Cell Dev Biol. 2020 May;101:104-110. doi: 10.1016/j.semcdb.2019.12.008. Epub 2019 Dec 24. Semin Cell Dev Biol. 2020. PMID: 31879264 Free PMC article. Review.
Cited by
- IL-10 deficiency aggravates cell senescence and accelerates BLM-induced pulmonary fibrosis in aged mice via PTEN/AKT/ERK pathway.
Li Y, Yin H, Yuan H, Wang E, Wang C, Li H, Geng X, Zhang Y, Bai J. Li Y, et al. BMC Pulm Med. 2024 Sep 11;24(1):443. doi: 10.1186/s12890-024-03260-x. BMC Pulm Med. 2024. PMID: 39261827 Free PMC article. - Cellular and Molecular Genetic Mechanisms of Lung Fibrosis Development and the Role of Vitamin D: A Review.
Enzel D, Kriventsov M, Sataieva T, Malygina V. Enzel D, et al. Int J Mol Sci. 2024 Aug 16;25(16):8946. doi: 10.3390/ijms25168946. Int J Mol Sci. 2024. PMID: 39201632 Free PMC article. Review. - Endometrial senescence is mediated by interleukin 17 receptor B signaling.
Kawamura K, Matsumura Y, Kawamura T, Araki H, Hamada N, Kuramoto K, Yagi H, Onoyama I, Asanoma K, Kato K. Kawamura K, et al. Cell Commun Signal. 2024 Jul 15;22(1):363. doi: 10.1186/s12964-024-01740-5. Cell Commun Signal. 2024. PMID: 39010112 Free PMC article. - The Role of Aging and Senescence in Immune Checkpoint Inhibitor Response and Toxicity.
Jain SS, Burton Sojo G, Sun H, Friedland BN, McNamara ME, Schmidt MO, Wellstein A. Jain SS, et al. Int J Mol Sci. 2024 Jun 27;25(13):7013. doi: 10.3390/ijms25137013. Int J Mol Sci. 2024. PMID: 39000121 Free PMC article. Review. - Stimuli-Specific Senescence of Primary Human Lung Fibroblasts Modulates Alveolar Stem Cell Function.
Melo-Narváez MC, Bramey N, See F, Heinzelmann K, Ballester B, Steinchen C, Jain E, Federl K, Hu Q, Dhakad D, Behr J, Eickelberg O, Yildirim AÖ, Königshoff M, Lehmann M. Melo-Narváez MC, et al. Cells. 2024 Jun 29;13(13):1129. doi: 10.3390/cells13131129. Cells. 2024. PMID: 38994981 Free PMC article.
References
- Metchnikoff E. The nature of man: studies in optimistic philosophy. G.P. Putnam's Sons Publishing; 1905.
- Ziesche R, Golec M, Samaha E. The RESOLVE concept: Approaching pathophysiology of fibroproliferative disease in aged individuals. Biogerontology. 2013;14:679–685. - PubMed
- Boorsma CE, Dekkers BG, van Dijk EM, Kumawat K, Richardson J, Burgess JK, John AE. Beyond TGFbeta--novel ways to target airway and parenchymal fibrosis. Pulm Pharmacol Ther. 2014;29:166–180. - PubMed
- Leung J, Cho Y, Lockey RF, Kolliputi N. The role of aging in idiopathic pulmonary fibrosis. Lung. 2015 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous