The exosomes in tumor immunity - PubMed (original) (raw)
Review
The exosomes in tumor immunity
Yanfang Liu et al. Oncoimmunology. 2015.
Abstract
Exosomes are a kind of nanometric membrane vesicles and can be released by almost all kinds of cells, including cancer cells. As the important mediators in intercellular communications, exosomes mediate exchange of protein and genetic material derived from parental cells. Emerging evidences show that exosomes secreted by either host cells or cancer cells are involved in tumor initiation, growth, invasion and metastasis. Moreover, communications between immune cells and cancer cells via exosomes play dual roles in modulating tumor immunity. In this review, we focus on exosome-mediated immunosuppression via inhibition of antitumor responses elicited by immune cells (DCs, NK cells, CD4+ and CD8+ T cells, etc.) and induction of immunosuppressive or regulatory cell populations (MDSCs, Tregs and Bregs). Transfer of cytokines, microRNAs (miRNAs) and functional mRNAs by tumor-derived exosomes (TEXs) is crucial in the immune escape. Furthermore, exosomes secreted from several kinds of immune cells (DCs, CD4+ and CD8+ Tregs) also participate in immunosuppression. On the other hand, we summarize the current application of DC-derived and modified tumor-derived exosomes as tumor vaccines. The potential challenges about exosome-based vaccines for clinical application are also discussed.
Keywords: exosomes; extracellular vesicle; immunosuppression; intercellular communication; tumor immunity; tumor vaccine.
Figures
Figure 1.
Exosome-mediated immunosuppression of tumor immunity. Exosomes derived from cancer cells have been shown to be involved in the modulation of tumor immunity in various ways: (A) Inhibition of proliferation and differentiation of CD4+ T cells into Th1 and Th17, inhibition of cytotoxicity of CTLs, NK cells and macrophages, inhibition of differentiation and maturation of DCs. (B) Promotion of CD4+, CD8+ Tregs and Bregs generation, induction of myeloid precursor differentiation into MDSCs, alternative activation of macrophages.
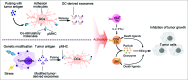
Figure 2.
Strategies for exosome-based tumor immunotherapy. This figure presents two main strategies for application of exosomes in tumor therapeutics. Exosomes secreted from antigen-loaded DCs carry functional peptide-MHC complexes, co-stimulatory and adhesion molecules and induce activation of CD4+ T cells, CD8+ T cells and NK cells, thus mediating cytotoxicity to tumor cells and inhibition of tumor growth. These molecules can also be exchanged between DCs to induce antitumor immune response indirectly. Additionally, tumor-derived exosomes (TEXs) carry tumor antigens and can trigger efficient antigen presentation of APCs, thus being used as resources of tumor antigens to prepare tumor vaccines. Moreover, modification of TEXs is developed to improve their immunogenicity, such as genetic engineering stress and protein loading.
Similar articles
- Exosome-Mediated Immunosuppression in Tumor Microenvironments.
Xie QH, Zheng JQ, Ding JY, Wu YF, Liu L, Yu ZL, Chen G. Xie QH, et al. Cells. 2022 Jun 16;11(12):1946. doi: 10.3390/cells11121946. Cells. 2022. PMID: 35741075 Free PMC article. Review. - Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis.
Hu S, Ma J, Su C, Chen Y, Shu Y, Qi Z, Zhang B, Shi G, Zhang Y, Zhang Y, Huang A, Kuang Y, Cheng P. Hu S, et al. Acta Biomater. 2021 Nov;135:567-581. doi: 10.1016/j.actbio.2021.09.003. Epub 2021 Sep 8. Acta Biomater. 2021. PMID: 34506976 - Dual effect of DLBCL-derived EXOs in lymphoma to improve DC vaccine efficacy in vitro while favor tumorgenesis in vivo.
Chen Z, You L, Wang L, Huang X, Liu H, Wei JY, Zhu L, Qian W. Chen Z, et al. J Exp Clin Cancer Res. 2018 Aug 13;37(1):190. doi: 10.1186/s13046-018-0863-7. J Exp Clin Cancer Res. 2018. PMID: 30103789 Free PMC article. - Enhancement of Anti-Leukemia Immunity by Leukemia-Derived Exosomes Via Downregulation of TGF-β1 Expression.
Huang F, Wan J, Hu W, Hao S. Huang F, et al. Cell Physiol Biochem. 2017;44(1):240-254. doi: 10.1159/000484677. Epub 2017 Nov 9. Cell Physiol Biochem. 2017. PMID: 29130994 - The function and clinical application of extracellular vesicles in innate immune regulation.
Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F. Zhou X, et al. Cell Mol Immunol. 2020 Apr;17(4):323-334. doi: 10.1038/s41423-020-0391-1. Epub 2020 Mar 19. Cell Mol Immunol. 2020. PMID: 32203193 Free PMC article. Review.
Cited by
- Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression.
Zhao K, Wang Z, Hackert T, Pitzer C, Zöller M. Zhao K, et al. J Exp Clin Cancer Res. 2018 Dec 12;37(1):312. doi: 10.1186/s13046-018-0961-6. J Exp Clin Cancer Res. 2018. PMID: 30541597 Free PMC article. Retracted. - Exosomes and pancreatic diseases: status, challenges, and hopes.
Guo XY, Xiao F, Li J, Zhou YN, Zhang WJ, Sun B, Wang G. Guo XY, et al. Int J Biol Sci. 2019 Jul 20;15(9):1846-1860. doi: 10.7150/ijbs.35823. eCollection 2019. Int J Biol Sci. 2019. PMID: 31523187 Free PMC article. Review. - Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis.
Li S, Pritchard DM, Yu LG. Li S, et al. Cancers (Basel). 2022 Jul 3;14(13):3263. doi: 10.3390/cancers14133263. Cancers (Basel). 2022. PMID: 35805035 Free PMC article. Review. - Hematological Malignancy-Derived Small Extracellular Vesicles and Tumor Microenvironment: The Art of Turning Foes into Friends.
Gargiulo E, Paggetti J, Moussay E. Gargiulo E, et al. Cells. 2019 May 27;8(5):511. doi: 10.3390/cells8050511. Cells. 2019. PMID: 31137912 Free PMC article. Review. - Tumor‑infiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer.
Xue Y, Tong L, LiuAnwei Liu F, Liu A, Zeng S, Xiong Q, Yang Z, He X, Sun Y, Xu C. Xue Y, et al. Oncol Rep. 2019 Aug;42(2):581-594. doi: 10.3892/or.2019.7196. Epub 2019 Jun 12. Oncol Rep. 2019. PMID: 31233191 Free PMC article.
References
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9:581-93; PMID:19498381; http://dx.doi.org/10.1038/nri2567 - DOI - PubMed
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013; 2:20389; PMID:24009890; http://dx.doi.org/10.3402/jev.v2i0.20389 - DOI - PMC - PubMed
- Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 2004; 164:1807-15; PMID:15111327; http://dx.doi.org/10.1016/S0002-9440(10)63739-X - DOI - PMC - PubMed
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 2010; 70:9621-30; PMID:21098712; http://dx.doi.org/10.1158/0008-5472.CAN-10-1722 - DOI - PubMed
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012; 30:1556-64; PMID:22605481; http://dx.doi.org/10.1002/stem.1129 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials