A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2015 Sep 25;10(9):e0138646.
doi: 10.1371/journal.pone.0138646. eCollection 2015.
Aurélie Cotillard 1, Camille Vatier 2, Jean-Philippe Bastard 3, Soraya Fellahi 3, Marie Stévant 4, Omran Allatif 2, Clotilde Langlois 5, Séverine Bieuvelet 4, Amandine Brochot 5, Angèle Guilbot 5, Karine Clément 1, Salwa W Rizkalla 1
Affiliations
- PMID: 26406981
- PMCID: PMC4583280
- DOI: 10.1371/journal.pone.0138646
Randomized Controlled Trial
A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial
Yuejun Liu et al. PLoS One. 2015.
Erratum in
- Correction: A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial.
Liu Y, Cotillard A, Vatier C, Bastard JP, Fellahi S, Stévant M, Allatif O, Langlois C, Bieuvelet S, Brochot A, Guilbot A, Clément K, Rizkalla SW. Liu Y, et al. PLoS One. 2015 Dec 14;10(12):e0145315. doi: 10.1371/journal.pone.0145315. eCollection 2015. PLoS One. 2015. PMID: 26661459 Free PMC article. No abstract available.
Abstract
Background: Preventing or slowing the progression of prediabetes to diabetes is a major therapeutic issue.
Objectives: Our aim was to evaluate the effects of 4-month treatment with a dietary supplement containing cinnamon, chromium and carnosine in moderately obese or overweight pre-diabetic subjects, the primary outcome being change in fasting plasma glucose (FPG) level. Other parameters of plasma glucose homeostasis, lipid profile, adiposity and inflammatory markers were also assessed.
Methods: In a randomized, double-blind, placebo-controlled study, 62 subjects with a FPG level ranging from 5.55 to 7 mmol/L and a body mass index ≥ 25 kg/m(2), unwilling to change their dietary and physical activity habits, were allocated to receive a 4-month treatment with either 1.2 g/day of the dietary supplement or placebo. Patients were followed up until 6 months post-randomization.
Results: Four-month treatment with the dietary supplement decreased FPG compared to placebo (-0.24 ± 0.50 vs +0.12 ± 0.59 mmol/L, respectively, p = 0.02), without detectable significant changes in HbA1c. Insulin sensitivity markers, plasma insulin, plasma lipids and inflammatory markers did not differ between the treatment groups. Although there were no significant differences in changes in body weight and energy or macronutrient intakes between the two groups, fat-free mass (%) increased with the dietary supplement compared to placebo (p = 0.02). Subjects with a higher FPG level and a milder inflammatory state at baseline benefited most from the dietary supplement.
Conclusions: Four-month treatment with a dietary supplement containing cinnamon, chromium and carnosine decreased FPG and increased fat-free mass in overweight or obese pre-diabetic subjects. These beneficial effects might open up new avenues in the prevention of diabetes.
Trial registration: ClinicalTrials.gov NCT01530685.
Conflict of interest statement
Competing Interests: PileJe (Saint-Laurent-des-Autels, France) provided funding towards this study. SB, AG and CL are employed by PiLeJe; AC received a grant from PiLeJe, OA and YL received fees for data management and other study procedures on the behalf of PiLeJe, MS is employed by AdipoPhYt at the time of the study. There are no patents or products in development to declare. The tested product was marketed before the beginning of the study. Other: CV has received consultancy fees from AstraZeneca and Sanofi, support for travel and congress inscription from Novonordisk and Sanofi, and research materials from AstraZeneca; JPB has received speaker fees from the European Group for the Study of Insulin Resistance (EGIR) and from Servier and a research grant from the Association Nationale de la Recherche sur le SIDA (ANRS); SF has received support for congress attendance from Beckman Coulter; the other authors declare no conflict of interest. The above declarations have no effect on the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
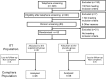
Fig 1. Consort flow diagram.
ITT: intention-to-treat. One subject in the dietary supplement group was lost to follow-up at Month 2, and one subject in the placebo group was withdrawn from the study at Month 2 due to a serious adverse event not related to study treatment (new condition with a need for hormonal treatment).
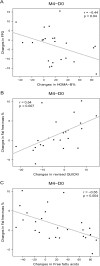
Fig 2. Correlations between changes in FPG or fat-free mass and other bioclinical parameters during dietary supplement treatment.
A: Correlation between changes in fasting plasma glucose and insulin secretion estimated by HOMA-B%. B: Correlations between changes in fat-free mass and changes in insulin sensitivity (estimated by revised QUICKI). C: Correlations between changes in fat-free mass and free fatty acids; N = 23 in A and B due to 3 missing data for plasma insulin, N = 26 subjects in C. Pearson correlations were used. D0: Day 0; M4: Month 4.
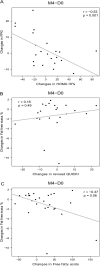
Fig 3. Correlations between changes in FPG or fat-free mass and other bioclinical parameters in the placebo group.
A: Correlation between changes in FPG and insulin secretion estimated by HOMA-B%. B: Correlations between changes in fat-free mass and changes in insulin sensitivity estimated by revised QUICKI. C: Correlations between changes in fat-free mass and free fatty acids; N = 22 in A and B due to 4 missing data for plasma insulin, N = 26 subjects in C. Pearson correlations were used. D0: Day 0; M4: Month 4.
Similar articles
- Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial.
Ranasinghe P, Galappaththy P, Constantine GR, Jayawardena R, Weeratunga HD, Premakumara S, Katulanda P. Ranasinghe P, et al. Trials. 2017 Sep 29;18(1):446. doi: 10.1186/s13063-017-2192-0. Trials. 2017. PMID: 28962661 Free PMC article. Clinical Trial. - Does supplementation with carnosine improve cardiometabolic health and cognitive function in patients with pre-diabetes and type 2 diabetes? study protocol for a randomised, double-blind, placebo-controlled trial.
Baye E, Menon K, de Courten MP, Earnest A, Cameron J, de Courten B. Baye E, et al. BMJ Open. 2017 Sep 1;7(9):e017691. doi: 10.1136/bmjopen-2017-017691. BMJ Open. 2017. PMID: 28864708 Free PMC article. Clinical Trial. - Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial.
Akilen R, Tsiami A, Devendra D, Robinson N. Akilen R, et al. Diabet Med. 2010 Oct;27(10):1159-67. doi: 10.1111/j.1464-5491.2010.03079.x. Diabet Med. 2010. PMID: 20854384 Clinical Trial. - Do Cinnamon Supplements Have a Role in Glycemic Control in Type 2 Diabetes? A Narrative Review.
Costello RB, Dwyer JT, Saldanha L, Bailey RL, Merkel J, Wambogo E. Costello RB, et al. J Acad Nutr Diet. 2016 Nov;116(11):1794-1802. doi: 10.1016/j.jand.2016.07.015. Epub 2016 Sep 8. J Acad Nutr Diet. 2016. PMID: 27618575 Free PMC article. Review. - To what extent does cinnamon administration improve the glycemic and lipid profiles?
Santos HO, da Silva GAR. Santos HO, et al. Clin Nutr ESPEN. 2018 Oct;27:1-9. doi: 10.1016/j.clnesp.2018.07.011. Epub 2018 Aug 13. Clin Nutr ESPEN. 2018. PMID: 30144878 Review.
Cited by
- Chromium Supplementation; Negotiation with Diabetes Mellitus, Hyperlipidemia and Depression.
Khodavirdipour A, Haddadi F, Keshavarzi S. Khodavirdipour A, et al. J Diabetes Metab Disord. 2020 Mar 5;19(1):585-595. doi: 10.1007/s40200-020-00501-8. eCollection 2020 Jun. J Diabetes Metab Disord. 2020. PMID: 32550211 Free PMC article. Review. - Use of carnosine in the prevention of cardiometabolic risk factors in overweight and obese individuals: study protocol for a randomised, double-blind placebo-controlled trial.
Menon K, Cameron JD, de Courten M, de Courten B. Menon K, et al. BMJ Open. 2021 May 13;11(5):e043680. doi: 10.1136/bmjopen-2020-043680. BMJ Open. 2021. PMID: 33986049 Free PMC article. - Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases.
Lucas K, Fröhlich-Nowoisky J, Oppitz N, Ackermann M. Lucas K, et al. Front Plant Sci. 2021 Feb 26;12:589783. doi: 10.3389/fpls.2021.589783. eCollection 2021. Front Plant Sci. 2021. PMID: 33719281 Free PMC article. No abstract available. - Effects of carnosine and histidine-containing dipeptides on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis.
Saadati S, Kabthymer RH, Aldini G, Mousa A, Feehan J, de Courten B. Saadati S, et al. Nutr Rev. 2024 Dec 1;82(12):1696-1709. doi: 10.1093/nutrit/nuad150. Nutr Rev. 2024. PMID: 38086332 Free PMC article. - Nutraceuticals and Supplements in Management of Prediabetes and Diabetes.
Derosa G, D'Angelo A, Angelini F, Belli L, Cicero AFG, Da Ros R, De Pergola G, Gaudio GV, Lupi A, Sartore G, Vignati FA, Maffioli P. Derosa G, et al. Nutrients. 2024 Dec 24;17(1):14. doi: 10.3390/nu17010014. Nutrients. 2024. PMID: 39796448 Free PMC article. Review.
References
- International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation; http://www.idf.org/diabetesatlas. 2013.
- Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–87. 10.1093/eurheartj/eht108 - DOI - PubMed
- Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14(7):933–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical