Clinical Outcome Assessments: Conceptual Foundation-Report of the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force - PubMed (original) (raw)
Practice Guideline
Clinical Outcome Assessments: Conceptual Foundation-Report of the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force
Marc K Walton et al. Value Health. 2015 Sep.
Abstract
An outcome assessment, the patient assessment used in an endpoint, is the measuring instrument that provides a rating or score (categorical or continuous) that is intended to represent some aspect of the patient's health status. Outcome assessments are used to define efficacy endpoints when developing a therapy for a disease or condition. Most efficacy endpoints are based on specified clinical assessments of patients. When clinical assessments are used as clinical trial outcomes, they are called clinical outcome assessments (COAs). COAs include any assessment that may be influenced by human choices, judgment, or motivation. COAs must be well-defined and possess adequate measurement properties to demonstrate (directly or indirectly) the benefits of a treatment. In contrast, a biomarker assessment is one that is subject to little, if any, patient motivational or rater judgmental influence. This is the first of two reports by the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force. This report provides foundational definitions important for an understanding of COA measurement principles. The foundation provided in this report includes what it means to demonstrate a beneficial effect, how assessments of patients relate to the objective of showing a treatment's benefit, and how these assessments are used in clinical trial endpoints. In addition, this report describes intrinsic attributes of patient assessments and clinical trial factors that can affect the properties of the measurements. These factors should be considered when developing or refining assessments. These considerations will aid investigators designing trials in their choice of using an existing assessment or developing a new outcome assessment. Although the focus of this report is on the development of a new COA to define endpoints in a clinical trial, these principles may be applied more generally. A critical element in appraising or developing a COA is to describe the treatment's intended benefit as an effect on a clearly identified aspect of how a patient feels or functions. This aspect must have importance to the patient and be part of the patient's typical life. This meaningful health aspect can be measured directly or measured indirectly when it is impractical to evaluate it directly or when it is difficult to measure. For indirect measurement, a concept of interest (COI) can be identified. The COI must be related to how a patient feels or functions. Procedures are then developed to measure the COI. The relationship of these measurements with how a patient feels or functions in the intended setting and manner of use of the COA (the context of use) could then be defined. A COA has identifiable attributes or characteristics that affect the measurement properties of the COA when used in endpoints. One of these features is whether judgment can influence the measurement, and if so, whose judgment. This attribute defines four categories of COAs: patient reported outcomes, clinician reported outcomes, observer reported outcomes, and performance outcomes. A full description as well as explanation of other important COA features is included in this report. The information in this report should aid in the development, refinement, and standardization of COAs, and, ultimately, improve their measurement properties.
Keywords: clinical outcome assessment; concept of interest; context of use; treatment benefit.
Copyright © 2015 International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. All rights reserved.
Figures
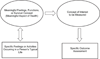
FIGURE 1. Relationship of Concepts to an Outcome Assessment
When planning a clinical trial investigators select a group of related feelings or activities that can occur in patient’s typical lives, are adversely affected by a disease, and are expected to show benefit from the intervention. The group of feelings or functions are identified as a specific conceptualized meaningful aspect of health. If the selected group of feelings or functions are not planned for direct measurement, a concept of interest that is thought measurable and substantially influences the meaningful aspect of health is formulated. The measurement of the concept of interest is operationalized as a specific, well defined, clinical outcome assessment. An interplay may occur between selection of the meaningful aspect of health and the concept of interest as investigators refine them to have a concept of interest for measurement that is thought both informative of the meaningful aspect of health and has a practical means to be measured (the specific clinical outcome assessment). In some cases the affected feelings or functions affected by the disease are intended to be directly measured, and the concept of interest for measurement is identical to the meaningful health aspect.
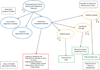
FIGURE 2. Relationships of Meaningful Functions to an Indirect Measure Outcome Assessment
A group of related activities meaningful to the patient (red rectangular box, Specific Meaningful Activities) are identified that would be a treatment benefit to the patient if improved by an intervention. A name is given to the meaningful aspect health that identifies the abstracted concept of the grouping (blue oval, Ambulation Dependent Function). This specific type of function is just one from among the range of meaningful areas of feeling or functioning (blue ovals) where patients with the disease might desire a treatment benefit. Each individual meaningful health aspect is a specific portion of the comprehensive range of all the meaningful feelings and functions affected by the disease any of which might be intended treatment benefit and evaluated in a clinical trial. In this example Ambulation Dependent function has been selected as the potential treatment benefit to be studied. Specific meaningful activities associated with the non-selected health aspects are not illustrated. Evaluation of the identified activities might be directly assessed in a defined outcome assessment (e.g., a PRO, not shown). The concept of interest for measurement in that case would be identical to the identified meaningful health aspect (see Figure 1). The selected meaningful health aspect might instead be deconstructed into narrowly defined concepts of interest that are thought to be important in performing the meaningful activities (COIs, orange ovals). Procedures could be devised as specified clinical outcome assessment (COA) instruments to represent a measurement of the COI (Defined Clinical Outcome Assessments, green boxes). In this example performance outcome tools (PerfO) are shown for each COI, but other PerfOs and other types of COAs could be developed for any of the COIs (see Figure 3). The PerfO procedure, an operationalized method to measure a body action identified as the COI, is a task not a part of a person’s usual normal life. The COA provides a score for the observed quality or quantity of performing the procedure and is used to form a study endpoint. The meaning of score or change in score to a person’s typical life is not intrinsically precisely known, but is hypothesized to reflect the meaningful functional activities. The actual meaning of a specific score (or change) to the patient cannot be known from the description of the PerfO procedure alone and should be evaluated in the process of developing the instrument. Dashed outline boxes name the type of concept or category of activity illustrated in that region of the figure, as presented in Figure 1. Ovals name specific concepts; either meaningful health aspects (blue) or COIs (orange). Solid outline boxes name specific functional activities of typical daily life that are meaningful (red box group), or specific procedures each of which might be developed as a COA (green boxes) to measure the selected COI. Short arrows not leading to a concept or a COA indicate that other possibilities exist, but are not shown.
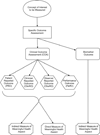
FIGURE 3. Attributes of Outcome Assessments
A specific outcome assessment is selected or created to operationalize measurement of the concept of interest. Outcome assessments are of two major types, clinical outcome assessments and biomarkers. Clinical outcome assessments have an attribute identifying the type of person whose judgment can influence the reported measurement. Clinical outcome assessments may be influenced by the judgment of the patient, clinician, or a non-clinician observer; they may also be a non-judged recording of task performed by the patient (performance outcome). Clinical outcome assessments may be directly reporting the meaningful feelings or functions selected as the potential treatment benefit, or may be reporting measurements that are thought to be indirectly informative regarding those feelings or functions (see Figure 1). Biomarkers can only indirectly measure the meaningful aspect of health.
Comment in
Similar articles
- Clinician-Reported Outcome Assessments of Treatment Benefit: Report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force.
Powers JH 3rd, Patrick DL, Walton MK, Marquis P, Cano S, Hobart J, Isaac M, Vamvakas S, Slagle A, Molsen E, Burke LB. Powers JH 3rd, et al. Value Health. 2017 Jan;20(1):2-14. doi: 10.1016/j.jval.2016.11.005. Epub 2017 Jan 10. Value Health. 2017. PMID: 28212963 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Patient-Reported Outcome and Observer-Reported Outcome Assessment in Rare Disease Clinical Trials: An ISPOR COA Emerging Good Practices Task Force Report.
Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Benjamin K, et al. Value Health. 2017 Jul-Aug;20(7):838-855. doi: 10.1016/j.jval.2017.05.015. Value Health. 2017. PMID: 28712612 - Consensus Recommendations for Clinical Outcome Assessments and Registry Development in Ataxias: Ataxia Global Initiative (AGI) Working Group Expert Guidance.
Klockgether T, Synofzik M; AGI working group on COAs and Registries. Klockgether T, et al. Cerebellum. 2024 Jun;23(3):924-930. doi: 10.1007/s12311-023-01547-z. Epub 2023 Apr 5. Cerebellum. 2024. PMID: 37020147 Free PMC article. Review.
Cited by
- Ataxia Rating Scales Reflect Patient Experience: an Examination of the Relationship Between Clinician Assessments of Cerebellar Ataxia and Patient-Reported Outcomes.
Potashman MH, Mize ML, Beiner MW, Pierce S, Coric V, Schmahmann JD. Potashman MH, et al. Cerebellum. 2023 Dec;22(6):1257-1273. doi: 10.1007/s12311-022-01494-1. Epub 2022 Dec 10. Cerebellum. 2023. PMID: 36495470 Free PMC article. - Patient-reported outcome measures in MS: Do development processes and patient involvement support valid quantification of clinically important variables?
Bharadia T, Vandercappellen J, Chitnis T, Eelen P, Bauer B, Brichetto G, Lloyd A, Schmidt H, King M, Fitzgerald J, Hach T, Hobart J. Bharadia T, et al. Mult Scler J Exp Transl Clin. 2022 Jun 22;8(2):20552173221105642. doi: 10.1177/20552173221105642. eCollection 2022 Apr-Jun. Mult Scler J Exp Transl Clin. 2022. PMID: 35755007 Free PMC article. - Patient-Centered Core Impact Sets: What They are and Why We Need Them.
Perfetto EM, Oehrlein EM, Love TR, Schoch S, Kennedy A, Bright J. Perfetto EM, et al. Patient. 2022 Nov;15(6):619-627. doi: 10.1007/s40271-022-00583-x. Epub 2022 Jun 2. Patient. 2022. PMID: 35653038 Free PMC article. - A pragmatic patient-reported outcome strategy for rare disease clinical trials: application of the EORTC item library to myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia.
Bell JA, Galaznik A, Pompilus F, Strzok S, Bejar R, Scipione F, Fram RJ, Faller DV, Cano S, Marquis P. Bell JA, et al. J Patient Rep Outcomes. 2019 Jun 19;3(1):35. doi: 10.1186/s41687-019-0123-4. J Patient Rep Outcomes. 2019. PMID: 31218454 Free PMC article. - Patient-Reported Outcome Measures for Patients Undergoing Inguinal Hernia Repair.
Gram-Hanssen A, Tolstrup A, Zetner D, Rosenberg J. Gram-Hanssen A, et al. Front Surg. 2020 Apr 16;7:17. doi: 10.3389/fsurg.2020.00017. eCollection 2020. Front Surg. 2020. PMID: 32373624 Free PMC article. Review.
References
- Johansson GM, Hager CK. Measurement properties of the Motor Evaluation Scale for Upper Extremity in Stroke patients (MESUPES) Disabil Rehabil. 2012;34:288–294. - PubMed
- Coster WJ, Haley SM, Ludlow LH, et al. Development of an applied cognition scale to measure rehabilitation outcomes. Arch Phys Med Rehabil. 2004;85:2030–2035. - PubMed
- Burk K, Schulz SR, Schulz JB. Monitoring progression in Friedreich ataxia (FRDA): the use of clinical scales. J Neurochem. 2013;126(Suppl. 1):118–124. - PubMed
- Howard K, Berry P, Petrillo J, et al. Development of the Shortness of Breath with Daily Activities questionnaire (SOBDA) Value Health. 2012;15:1042–1050. - PubMed
- Padua L, Evoli A, Aprile I, et al. Myasthenia gravis outcome measure: development and validation of a disease-specific self-administered questionnaire. Neurol Sci. 2012;23:59–68. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical