TRPV1 Channel: A Potential Drug Target for Treating Epilepsy - PubMed (original) (raw)
Review
TRPV1 Channel: A Potential Drug Target for Treating Epilepsy
Mustafa Nazıroğlu. Curr Neuropharmacol. 2015.
Abstract
Epilepsy has 2-3% incidence worldwide. However, present antiepileptic drugs provide only partial control of seizures. Calcium ion accumulation in hippocampal neurons has long been known as a major contributor to the etiology of epilepsy. TRPV1 is a calcium-permeable channel and mediator of epilepsy in the hippocampus. TRPV1 is expressed in epileptic brain areas such as CA1 area and dentate gyrus of the hippocampus. Here the author reviews the patent literature on novel molecules targeting TRPV1 that are currently being investigated in the laboratory and are candidates for future clinical evaluation in the management of epilepsy. A limited number of recent reports have implicated TRPV1 in the induction or treatment of epilepsy suggesting that this may be new area for potential drugs targeting this debilitating disease. Thus activation of TRPV1 by oxidative stress, resiniferatoxin, cannabinoid receptor (CB1) activators (i.e. anandamide) or capsaicin induced epileptic effects, and these effects could be reduced by appropriate inhibitors, including capsazepine (CPZ), 5'-iodoresiniferatoxin (IRTX), resolvins, and CB1 antagonists. It has been also reported that CPZ and IRTX reduced spontaneous excitatory synaptic transmission through modulation of glutaminergic systems and desensitization of TRPV1 channels in the hippocampus of rats. Immunocytochemical studies indicated that TRPV1 channel expression increased in the hippocampus of mice and patients with temporal lobe epilepsy. Taken together, findings in the current literature support a role for calcium ion accumulation through TRPV1 channels in the etiology of epileptic seizures, indicating that inhibition of TRPV1 in the hippocampus may possibly be a novel target for prevention of epileptic seizures.
Figures
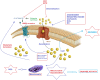
Fig. (1)
Possible molecular pathways of TRPV1 channel activation on epilepsy in hippocampal neurons. Convulsions in epilepsy can results in augmented glutamate release, leading to Ca2+ uptake through NMDA receptor and TRP channels. Mitochondria were reported to accumulate Ca2+ provided cytosolic Ca2+ rises, thereby leading to depolarization of mitochondrial membranes. At the extreme, Ca2+ entry causes severe mitochondrial permeability transition or even the rupture of the mitochondrial membrane, substantial swelling of the mitochondria with rupture of the outer membrane and release of apoptosis-inducing factors such as caspase 3 and 9. Anandamide, capsaicin, resiniferatoxin, nitric oxide (NO) and reactive oxygen species (ROS) induce Ca2+ accumulation through desensitization of TRPV1 channels although pharmacological desensitization of TRPV1 channels through antagonists such as capsazepine (CPZ) and 5'-iodoresiniferatoxin (IRTX) contributes to an immediate reduction on neuronal excitability [78]. ROS enhance also spontaneous release of glutamate from presynaptic terminals onto neurons through TRPV1 channel activation. The molecular pathway may be a cause of epileptic seizures and the subject should urgently investigate.
Similar articles
- Involvement of apoptosis and calcium accumulation through TRPV1 channels in neurobiology of epilepsy.
Nazıroğlu M, Övey İS. Nazıroğlu M, et al. Neuroscience. 2015 May 7;293:55-66. doi: 10.1016/j.neuroscience.2015.02.041. Epub 2015 Mar 2. Neuroscience. 2015. PMID: 25743251 - Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model.
Nazıroğlu M, Taner AN, Balbay E, Çiğ B. Nazıroğlu M, et al. Mol Cell Biochem. 2019 Mar;453(1-2):143-155. doi: 10.1007/s11010-018-3439-0. Epub 2018 Aug 29. Mol Cell Biochem. 2019. PMID: 30159798 - Electromagnetic radiation (Wi-Fi) and epilepsy induce calcium entry and apoptosis through activation of TRPV1 channel in hippocampus and dorsal root ganglion of rats.
Ghazizadeh V, Nazıroğlu M. Ghazizadeh V, et al. Metab Brain Dis. 2014 Sep;29(3):787-99. doi: 10.1007/s11011-014-9549-9. Epub 2014 May 3. Metab Brain Dis. 2014. PMID: 24792079 - TRPV1 channel in the pathophysiology of epilepsy and its potential as a molecular target for the development of new antiseizure drug candidates.
Socała K, Jakubiec M, Abram M, Mlost J, Starowicz K, Kamiński RM, Ciepiela K, Andres-Mach M, Zagaja M, Metcalf CS, Zawadzki P, Wlaź P, Kamiński K. Socała K, et al. Prog Neurobiol. 2024 Sep;240:102634. doi: 10.1016/j.pneurobio.2024.102634. Epub 2024 Jun 2. Prog Neurobiol. 2024. PMID: 38834133 Review. - Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection.
Rosenberg EC, Patra PH, Whalley BJ. Rosenberg EC, et al. Epilepsy Behav. 2017 May;70(Pt B):319-327. doi: 10.1016/j.yebeh.2016.11.006. Epub 2017 Feb 9. Epilepsy Behav. 2017. PMID: 28190698 Free PMC article. Review.
Cited by
- Identification of New Compounds with Anticonvulsant and Antinociceptive Properties in a Group of 3-substituted (2,5-dioxo-pyrrolidin-1-yl)(phenyl)-Acetamides.
Abram M, Jakubiec M, Rapacz A, Mogilski S, Latacz G, Szulczyk B, Szafarz M, Socała K, Nieoczym D, Wyska E, Wlaź P, Kamiński RM, Kamiński K. Abram M, et al. Int J Mol Sci. 2021 Dec 3;22(23):13092. doi: 10.3390/ijms222313092. Int J Mol Sci. 2021. PMID: 34884898 Free PMC article. - Synthesis, Anticonvulsant and Antinociceptive Activity of New Hybrid Compounds: Derivatives of 3-(3-Methylthiophen-2-yl)-pyrrolidine-2,5-dione.
Góra M, Czopek A, Rapacz A, Dziubina A, Głuch-Lutwin M, Mordyl B, Obniska J. Góra M, et al. Int J Mol Sci. 2020 Aug 11;21(16):5750. doi: 10.3390/ijms21165750. Int J Mol Sci. 2020. PMID: 32796594 Free PMC article. - Anesthetic- and Analgesic-Related Drugs Modulating Both Voltage-Gated Na+ and TRP Channels.
Kumamoto E. Kumamoto E. Biomolecules. 2024 Dec 18;14(12):1619. doi: 10.3390/biom14121619. Biomolecules. 2024. PMID: 39766326 Free PMC article. Review. - Green-Synthesized Silver Nanoparticles Using Filipendula ulmaria (L.) Maxim. and Salvia verticillata L. Extracts Inhibit Migration and Modulate Redox Homeostasis in Human Breast Cancer Cells via Nrf-2 Signaling Pathway.
Matić M, Obradović A, Paunović M, Ognjanović B, Mihailović V, Srećković N, Stanković M. Matić M, et al. Antioxidants (Basel). 2025 Apr 14;14(4):469. doi: 10.3390/antiox14040469. Antioxidants (Basel). 2025. PMID: 40298802 Free PMC article. - Beneficial Effects of Capsaicin in Disorders of the Central Nervous System.
Pasierski M, Szulczyk B. Pasierski M, et al. Molecules. 2022 Apr 12;27(8):2484. doi: 10.3390/molecules27082484. Molecules. 2022. PMID: 35458680 Free PMC article. Review.
References
- Iannotti F.A., Hill C.L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., Russo E., Whalley B.J., Di Marzo V., Stephens G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014;5(11):1131–1141. doi: 10.1021/cn5000524. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous