Crystal Structure of the DNA Deaminase APOBEC3B Catalytic Domain - PubMed (original) (raw)
Crystal Structure of the DNA Deaminase APOBEC3B Catalytic Domain
Ke Shi et al. J Biol Chem. 2015.
Abstract
Functional and deep sequencing studies have combined to demonstrate the involvement of APOBEC3B in cancer mutagenesis. APOBEC3B is a single-stranded DNA cytosine deaminase that functions normally as a nuclear-localized restriction factor of DNA-based pathogens. However, it is overexpressed in cancer cells and elicits an intrinsic preference for 5'-TC motifs in single-stranded DNA, which is the most frequently mutated dinucleotide in breast, head/neck, lung, bladder, cervical, and several other tumor types. In many cases, APOBEC3B mutagenesis accounts for the majority of both dispersed and clustered (kataegis) cytosine mutations. Here, we report the first structures of the APOBEC3B catalytic domain in multiple crystal forms. These structures reveal a tightly closed active site conformation and suggest that substrate accessibility is regulated by adjacent flexible loops. Residues important for catalysis are identified by mutation analyses, and the results provide insights into the mechanism of target site selection. We also report a nucleotide (dCMP)-bound crystal structure that informs a multistep model for binding single-stranded DNA. Overall, these high resolution crystal structures provide a framework for further mechanistic studies and the development of novel anti-cancer drugs to inhibit this enzyme, dampen tumor evolution, and minimize adverse outcomes such as drug resistance and metastasis.
Keywords: APOBEC3B; DNA binding protein; DNA deaminase; DNA editing; DNA repair; Mutation; cancer; cancer mutagenesis; crystal structure; enzyme mutation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
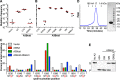
FIGURE 1.
Generation of an active and soluble A3B catalytic domain construct. A and B, rifampicin resistance (rpoB) mutation frequency of E. coli expressing the indicated Sumo-A3B constructs. Each dot represents data from an individual culture (n = 5 per condition), and the horizontal line reports the median value for each condition. Vector, pE-Sumo-Amp; WT, full-length wild-type A3B; CTD, A3Bctd residues 187–378. Amino acid substitution mutants are derivatives of A3Bctd. C, rifampicin resistance (rpoB) mutation spectrum of E. coli expressing WT, A3Bctd, and A3Bctd-QMΔloop3 in comparison to the vector control. A histogram summarizing the percentage of C to T transitions occurring at mutable positions in rpoB (shown in tetranucleotide context with mutated base colored red, underlined, and numbered according to position in the rpoB gene). D, size exclusion chromatography profile of purified A3Bctd-QMΔloop3 and Coomassie Brilliant Blue-stained SDS-PAGE analysis of purified A3Bctd-QMΔloop3. The protein concentration was adjusted to 1.5 mg ml−1 prior to injection into a Superdex 200 10/300 GL column. The molecular mass standards used were as follows; bovine γ-globulin (160 kDa), chicken ovalbumin (44 kDa), horse myoglobin (17 kDa), and vitamin B12 (1.4 kDa). E, comparison of the in vitro DNA deaminase activities of recombinant A3A purified from 293 cells and 10 μ
m
A3Bctd-DM, A3Bctd-QM, and A3Bctd-QMΔloop3 purified from E. coli. S, substrate; P, product.
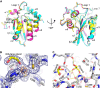
FIGURE 2.
A3B catalytic domain structures. A, superposition of three independent A3Bctd-QMΔloop3 structures. The structure refined in the orthorhombic space group (P212121) is in cyan, and the two crystallographically independent molecules in the monoclinic space group (P21) are shown in magenta and yellow. A gray sphere represents each zinc ion. B, A3B active site residues involved in zinc coordination. The fourth ligand of zinc coordination in this case is ethylene glycol (yellow and red). The final model is overlaid with the simulated annealing, composite omit 2_F_o − _F_c map contoured at 1.0 σ (blue mesh) or 10.0 σ (orange mesh). C, the network of interactions in the glycerol-occupied active site pocket. Hydrogen bonds are shown in yellow, and the zinc-coordinating interactions are shown as magenta dotted lines. The fourth ligand of zinc coordination in this case is a water molecule (hydroxyl ion).
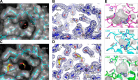
FIGURE 3.
The closed conformation of the A3B active site. A, molecular surface around the active site of A3Bctd-QMΔloop3 showing a small portal into the catalytic pocket. The active site pocket contains ordered water molecules represented by red spheres and an ethylene glycol molecule in yellow as shown in Fig. 2_B. B_, electron density for the region shown in A. The final model is overlaid with the simulated annealing, composite omit 2_F_o − F_c map as in Fig. 2_B. C, a view of the active site pocket with glycerol molecules shown in yellow and red sticks. D, electron density for the region shown in C. The simulated annealing composite omit 2_F_o − _F_c map is contoured at 1.0 σ (blue mesh). E, comparison of active site conformations for A3A (PDB code 4XXO), A3Bctd-QMΔloop3 (this study), and A3Gctd (PDB code 3V4K). The gray wireframes show pockets/cavities in each protein.
FIGURE 4.
Enzymatic activity and local preferences in E. coli. A, rifampicin resistance (rpoB) mutation frequencies of E. coli expressing the indicated Sumo-A3Bctd constructs (active site single amino acid substitution mutants). Performed and labeled as described for experiments in Fig. 1. B, rifampicin resistance (rpoB) mutation frequency of E. coli expressing the indicated chimeric A3B constructs. Each dot represents data from an individual culture (n = 5 per condition), and the horizontal line reports the median value for each condition. C, comparison of the in vitro DNA deaminase activities of purified A3Bctd-QMΔloop3 and A3Bctd-QMΔloop3-A3Aloop1 over the indicated ranges of protein concentrations (in μ
m
). S, substrate; P, product. D, rifampicin resistance (rpoB) mutation spectra for select constructs from C. The histogram summarizes the percentage of C to T transitions occurring at mutable positions in the rpoB gene shown in tetranucleotide context with mutated base colored red, underlined, and numbered according to position in the coding sequence. To facilitate comparisons, the vector, A3Bctd, and A3Bctd-QMΔL3 data are reproduced from Fig. 1.
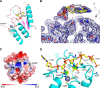
FIGURE 5.
A dCMP-bound structure and a model for A3Bctd-ssDNA complex formation. A, a ribbon schematic of A3Bctd-QMΔloop3 with dCMP bound on the protein surface shown as sticks. B, a close-up view of the bound dCMP molecule (yellow) and the interacting protein residues (gray) as sticks, overlaid with the simulated annealing, composite omit 2_F_o − _F_c map contoured at 1.0 σ (blue mesh). C, A3Bctd-QMΔloop3 surface colored according to the electrostatic potential (−5.0 to +5.0 kT/e), with the bound dCMP as sticks. A3Bctd-QMΔloop3 is oriented to position the active site pocket approximately in the middle of the view. D, a hypothetical model of A3Bctd bound to a 5-mer ssDNA, based on the crystallographically observed nucleotide. See main text for details.
Similar articles
- Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B.
Shi K, Carpenter MA, Banerjee S, Shaban NM, Kurahashi K, Salamango DJ, McCann JL, Starrett GJ, Duffy JV, Demir Ö, Amaro RE, Harki DA, Harris RS, Aihara H. Shi K, et al. Nat Struct Mol Biol. 2017 Feb;24(2):131-139. doi: 10.1038/nsmb.3344. Epub 2016 Dec 19. Nat Struct Mol Biol. 2017. PMID: 27991903 Free PMC article. - Conformational Switch Regulates the DNA Cytosine Deaminase Activity of Human APOBEC3B.
Shi K, Demir Ö, Carpenter MA, Wagner J, Kurahashi K, Harris RS, Amaro RE, Aihara H. Shi K, et al. Sci Rep. 2017 Dec 12;7(1):17415. doi: 10.1038/s41598-017-17694-3. Sci Rep. 2017. PMID: 29234087 Free PMC article. - Polyomavirus T Antigen Induces APOBEC3B Expression Using an LXCXE-Dependent and TP53-Independent Mechanism.
Starrett GJ, Serebrenik AA, Roelofs PA, McCann JL, Verhalen B, Jarvis MC, Stewart TA, Law EK, Krupp A, Jiang M, Martens JWM, Cahir-McFarland E, Span PN, Harris RS. Starrett GJ, et al. mBio. 2019 Feb 5;10(1):e02690-18. doi: 10.1128/mBio.02690-18. mBio. 2019. PMID: 30723127 Free PMC article. - Molecular mechanism and clinical impact of APOBEC3B-catalyzed mutagenesis in breast cancer.
Harris RS. Harris RS. Breast Cancer Res. 2015 Jan 21;17(1):8. doi: 10.1186/s13058-014-0498-3. Breast Cancer Res. 2015. PMID: 25848704 Free PMC article. Review. - APOBEC3B, a molecular driver of mutagenesis in human cancers.
Zou J, Wang C, Ma X, Wang E, Peng G. Zou J, et al. Cell Biosci. 2017 May 30;7:29. doi: 10.1186/s13578-017-0156-4. eCollection 2017. Cell Biosci. 2017. PMID: 28572915 Free PMC article. Review.
Cited by
- Targeting natural splicing plasticity of APOBEC3B restricts its expression and mutagenic activity.
Rouf Banday A, Onabajo OO, Lin SH, Obajemu A, Vargas JM, Delviks-Frankenberry KA, Lamy P, Bayanjargal A, Zettelmeyer C, Florez-Vargas O, Pathak VK, Dyrskjøt L, Prokunina-Olsson L. Rouf Banday A, et al. Commun Biol. 2021 Mar 22;4(1):386. doi: 10.1038/s42003-021-01844-5. Commun Biol. 2021. PMID: 33753867 Free PMC article. - The current toolbox for APOBEC drug discovery.
Grillo MJ, Jones KFM, Carpenter MA, Harris RS, Harki DA. Grillo MJ, et al. Trends Pharmacol Sci. 2022 May;43(5):362-377. doi: 10.1016/j.tips.2022.02.007. Trends Pharmacol Sci. 2022. PMID: 35272863 Free PMC article. Review. - BE-FLARE: a fluorescent reporter of base editing activity reveals editing characteristics of APOBEC3A and APOBEC3B.
Coelho MA, Li S, Pane LS, Firth M, Ciotta G, Wrigley JD, Cuomo ME, Maresca M, Taylor BJM. Coelho MA, et al. BMC Biol. 2018 Dec 28;16(1):150. doi: 10.1186/s12915-018-0617-1. BMC Biol. 2018. PMID: 30593278 Free PMC article. - APOBEC Reporter Systems for Evaluating diNucleotide Editing Levels.
Rieffer AE, Chen Y, Salamango DJ, Moraes SN, Harris RS. Rieffer AE, et al. CRISPR J. 2023 Oct;6(5):430-446. doi: 10.1089/crispr.2023.0027. Epub 2023 Sep 6. CRISPR J. 2023. PMID: 37672599 Free PMC article. - APOBEC Enzymes as Targets for Virus and Cancer Therapy.
Olson ME, Harris RS, Harki DA. Olson ME, et al. Cell Chem Biol. 2018 Jan 18;25(1):36-49. doi: 10.1016/j.chembiol.2017.10.007. Epub 2017 Nov 16. Cell Chem Biol. 2018. PMID: 29153851 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103403/GM/NIGMS NIH HHS/United States
- R37 AI064046/AI/NIAID NIH HHS/United States
- GM091743/GM/NIGMS NIH HHS/United States
- AI064046/AI/NIAID NIH HHS/United States
- R56 AI064046/AI/NIAID NIH HHS/United States
- GM110734/GM/NIGMS NIH HHS/United States
- GM109770/GM/NIGMS NIH HHS/United States
- Howard Hughes Medical Institute/United States
- GM095558/GM/NIGMS NIH HHS/United States
- R01 GM109770/GM/NIGMS NIH HHS/United States
- P01 GM091743/GM/NIGMS NIH HHS/United States
- R01 GM110734/GM/NIGMS NIH HHS/United States
- R01 AI064046/AI/NIAID NIH HHS/United States
- R01 GM095558/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources